|
 |
- Search
| J Acute Care Surg > Volume 12(1); 2022 > Article |
|
Abstract
A thyroid storm is a rare complication of hyperthyroidism. Although a thyroid storm rarely presents with symptoms similar to those of an acute abdomen, and in cases where emergency surgery is needed, the thyroid function test is not performed routinely. In this study, we report a case in which hyperthyroidism was diagnosed after surgery in a patient with recurrent refractory peptic ulcer disease. Although peptic ulcer disease and hyperthyroidism rarely coexist, when the patient’s initial condition was reviewed in the Emergency Department, the findings were reasonable for panperitonitis due to peptic ulcer perforation, which is considered as a condition suitable for a thyroid storm. This isolated case indicates a logical leap in the correlation between peptic ulcer and thyroid storm. In recurrent refractory peptic ulcer disease, the thyroid function test may be helpful as a routine laboratory test before emergency surgery.
A thyroid storm is a rare complication of hyperthyroidism. It often occurs during thyroid manipulation in thyroid surgery, therefore, prevention is necessary before thyroid surgery in patients with hyperthyroidism [1]. Thyrotoxicosis and thyroid storm rarely present with symptoms similar to those of acute abdomen [2,3]. However, hyperthyroidism, as a result of Graves’ disease, is easily overlooked in patients undergoing emergency surgery for an acute abdomen such as bowel perforation. To date, the exact causal relationship between hyperthyroidism and peptic ulcers has not been elucidated. In addition, patients with peptic ulcer accompanied by hyperthyroidism are very rare, and the number of published cases is very limited [4–7]. In this study, we report a case of Graves’ disease observed as an isolated case of a patient with multiple peptic ulcers.
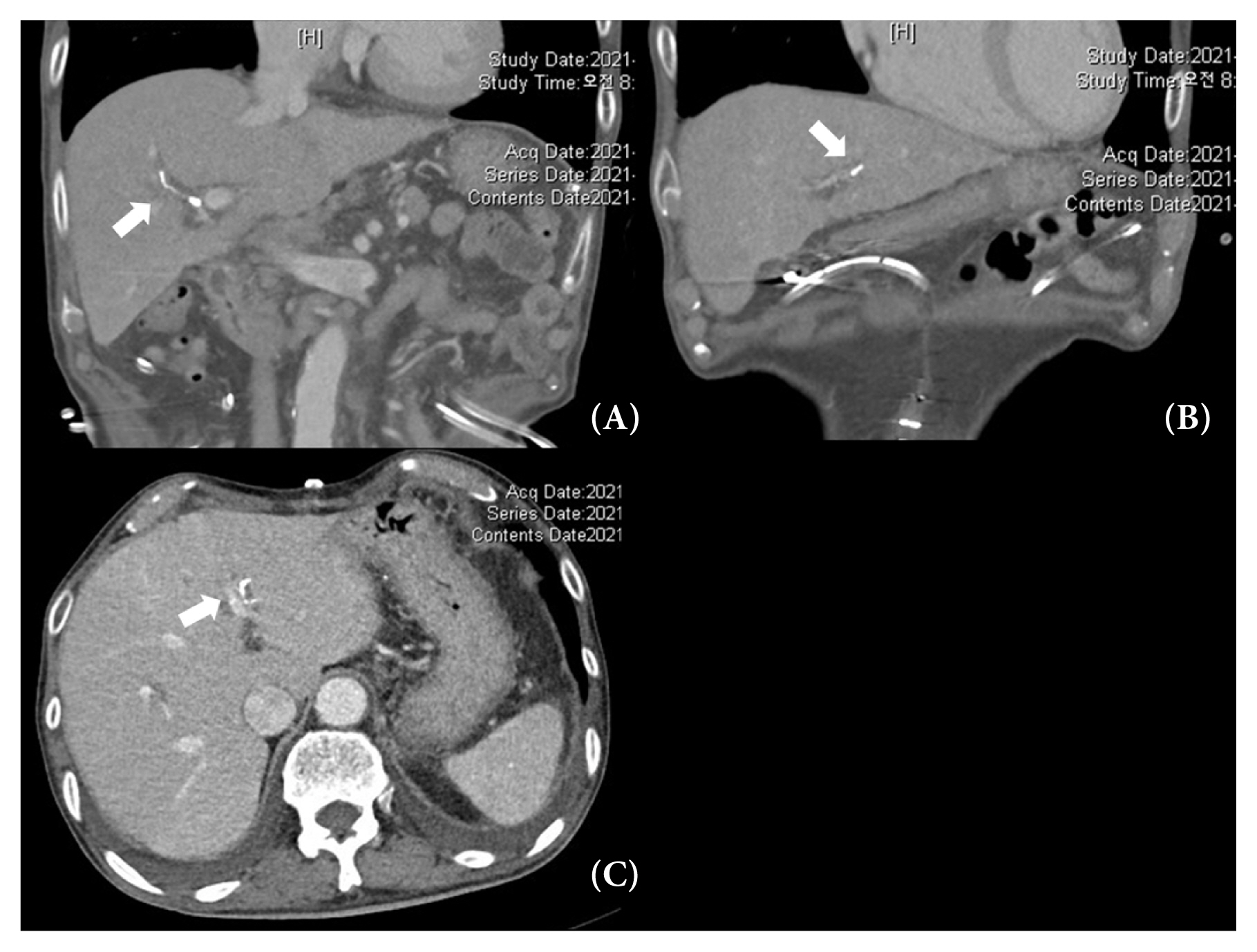
A 64-year-old male with no specific history visited the emergency room (ER) of the National Health Insurance Service Ilsan Hospital with abdominal pain and hematochezia that had been aggravated for 2 days. The patient’s body mass index was 16.51. The patient’s vital signs at arrival to the ER were a systolic blood pressure of 188 mmHg, a diastolic blood pressure of 75 mmHg, a heart rate (HR) of 128 bpm, a respiratory rate of 22 breaths per minute, and a body temperature of 37.7°C. Laboratory findings for the patient were a hemoglobin (Hb) level of 8.9 g/dL, white blood cell count of 29,580/uL, a segmented neutrophil rate of 92.3%, a C-reactive protein level of 5.75 mg/dL, a total bilirubin level of 5.78 mg/dL and direct bilirubin 4.09 mg/dL, an alkaline phosphatase level of 151 IU/L, a gamma-GT level of 82 IU/L, a lactate level of 5.5 mmol/L, and a procalcitonin concentration of 2.28 ng/mL. The Sequential Organ Failure Assessment score was 3 points. The patient underwent an esophagogastroduodenoscopy (EGD) and a colonoscopy 1 week before admission, and the findings were normal. On physical examination, direct tenderness was observed throughout the abdomen, and the abdomen was generally rigid. Abdominopelvic computed tomography was performed for further evaluation. A perforation was found in the 1st portion of the duodenum, and there was free air and fluid collection at the periperforation site (Figure 1). The decision was made to perform an emergency exploratory laparotomy. Operating room findings revealed there was a perforation which was approximately 3 mm, in the 1st portion of the duodenum. A primary repair and omentopexy were performed.
Subsequently, the patient seemed to recover, however, the level of Hb decreased with the passage of a small amount of melena on the 3rd day after surgery. Transfusion and hemostatic agents were administered, however, severe hematochezia was observed with a decrease in blood pressure on the 7th day after surgery. Emergency computed tomography angiography revealed active bleeding of the gastroduodenal artery, and embolization was performed. The patient then stabilized without additional transfusions.
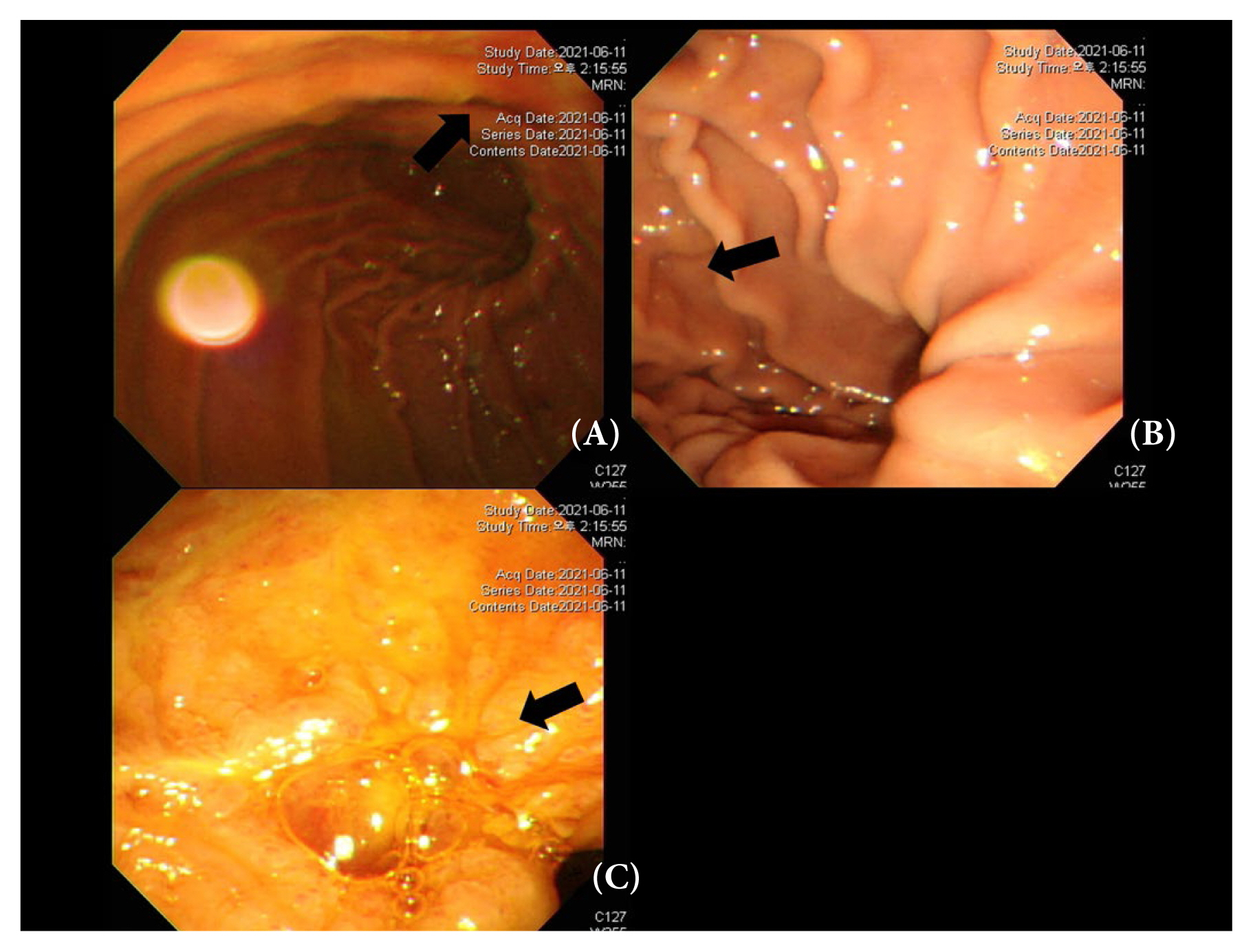
The patient had hyperbilirubinemia (5.78 mg/dL) upon arrival at the ER, and the levels of bilirubin continued to rise after embolization of the gastroduodenal artery. For a differential diagnosis, an ampullotomy and stone removal were performed using endoscopic retrograde cholangiopancreatography. However, the total bilirubin level increased to 24 mg/dL and the direct bilirubin level was 16 mg/dL. When an additional computed tomography scan was performed, the cause of the hyperbilirubinemia was considered to be liver dysfunction due to the glue used for embolization (Figure 2). Subsequently, the bilirubin levels decrease to 8.0 mg/dL without any other measures. A follow-up EGD was performed on the 14th day after surgery, and another ulcer (near the duodenal bulb) was discovered (Figure 3). However, it was in a state of healing. The patient was discharged on the 20th day after surgery with medication for ulcer treatment.
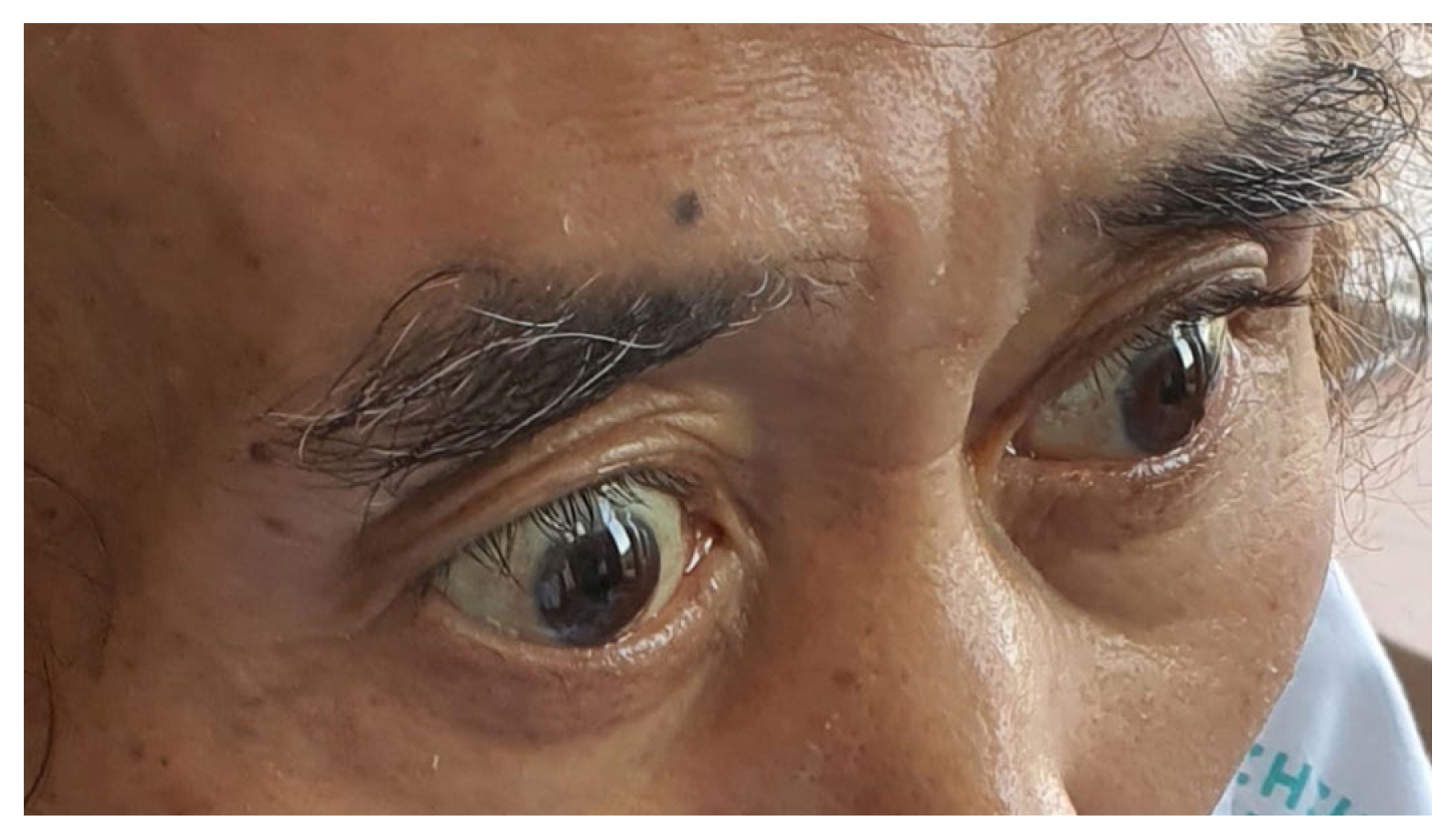
The patient visited the outpatient clinic on the 32nd day after surgery and complained of general weakness, poor oral intake, hematochezia, and hyperbilirubinemia. He was readmitted for supportive care. A computed tomography angiography scan revealed aggravation of the ulcer near the duodenal bulb. In addition, a large amount of hematochezia with a decrease in Hb levels was observed, and a blood transfusion was performed. However, due to concerns about iatrogenic damage, an EGD was not performed. Ocular protrusion was observed, which was thought to be exophthalmos caused by hyperthyroidism, and a thyroid function test was performed (Figure 4). The thyroid function tests [T3 151 ng/dL, free T4 4.93 ng/dL, TSH < 0.01, thyroglobulin Ab 716 IU/mL, TSH-receptor Ab > 40.00 IU/L, and an anti-microsomal antibody (TPO Ab) was positive] were indicative of Graves’ disease. The patient recovered following the administration of methimazole and was discharged on the 52nd day after surgery.
In this study, we report a case of recurrent refractory peptic ulcer with hyperthyroidism. Notably, the patient underwent an EGD 10 days before admission to the ER and there were no special findings, but there was an ulcer perforation at the time of admission. Aggressive factors for peptic ulcer include hydrochloric acid secretion, pepsin, ethanol ingestion, smoking, duodenal reflux of bile, ischemia, non-steroidal anti-inflammatory drugs, hypoxia, and a Helicobacter pylori infection [1]. In the past, peptic ulcer disease and hyperthyroidism were considered as conditions which did not coexist. This is because peptic ulcer disease is a parasympathetic dominant disease, whereas hyperthyroidism is a sympathetic dominant disease [8,9]. In addition, in a study of 9,618 patients with hyperthyroidism, there was no significant association between hyperthyroidism and peptic ulcers [6]. Treatment with methimazole and beta blockers for hyperthyroidism increased gastric secretion in patients, leading to peptic ulcers [10]. In addition, an animal study showed that increased levels of thyroid hormones increased the thickness of the gastric mucosa, thereby adding to the treatment of ulcers [11]. However, there have been discrepant studies. In a study, hyperthyroidism caused delayed gastric emptying and increased histological gastritis. Another study on thyrotoxicosis determined that the incidence of duodenal ulcers increased regardless of the level of gastric acid production and gastric emptying [4,5,8]. Moreover, another study described a case of thyroid storm induced by duodenal ulcer perforation in a patient who had already been treated with methimazole for Graves’ disease.
Thyroid storms are caused by a sudden increase in the levels of thyroid hormones T3 and T4, which usually occurs 8 to 16 hours after surgery [12,13]. Therefore, patients with a history of hyperthyroidism should maintain their euthyroid status by administering thionamides such as methimazole and propylthiouracil before surgery to prevent a postoperative thyroid storm [14,15]. However, in the case of emergency surgery, premedication is not usually administered in the ER or on the wards. Therefore, if hyperthyroidism is suspected, but the history is unknown, the patient should be consulted with the anesthesiologist prior to surgery to administer barbiturate and benzodiazepine during surgery, and to avoid using anticholinergic drugs and sympathetic stimulants [13,15].
The National Health Insurance Service Ilsan Hospital does not routinely use thyroid function tests before emergency operations are performed. Initially, the patient presented with a low body mass index, and agitation upon arrival at the ER, but hyperthyroidism was not suspected. Therefore, it is not clear whether hyperthyroidism was aggravated by duodenal ulcer perforation or whether the peptic ulcer was aggravated by hyperthyroidism. Assessment on the 1st day in the ER, the patient was agitated and had a HR of 128 bpm, a respiratory rate of 22 breaths per minute, a body temperature of 37.7°C, atrial fibrillation, and unexplained hyperbilirubinemia. Consequently, the Burch-Wartofsky Point Scale (BWPS) was used to determine whether the patient was experiencing a thyroid storm. A score of 45 or higher indicates thyroid storm and the patient’s BWPS score was 60. Therefore, thyroid storm could not be ruled out [16]. However, this set of conditions was similar to those used for the diagnosis of panperitonitis. Hence, interpreting it as a thyroid storm was a logical leap. Upon readmission, the patient’s BWPS was 40 with an HR of 107 bpm, a body temperature of 37.2°C, atrial fibrillation, and hyperbilirubinemia. In such cases, a thyroid storm should also be considered. It is important to consider the cause of the patient’s hyperbilirubinemia. Since hyperbilirubinemia in this case could be due to partial liver damage caused by embolization, it may be difficult to consider hyperthyroidism as the cause. However, since hyperbilirubinemia was present at the time of the initial arrival at the ER, it is difficult to determine the exact cause because it is extremely rare in patients with hyperthyroidism, and it is most likely caused by cholestasis occurring in thyrotoxicosis [17]. It has been hypothesized that cholestasis occurs as bile transport is interrupted due to increased hepatic oxygen demand without increased blood flow due to hyperthyroidism [17–19]. Another hypothesis is that thyroxine is directly toxic to hepatocytes, however, none of these hypotheses have been proven [19]. Therefore, if the patient had hyperthyroidism and peptic ulcer disease, as in this case, the cause of jaundice may be considered as hyperthyroidism.
In this case report, the possibility of aggravation of peptic ulcer disease was shown in a patient with hyperthyroidism. Since a patient is usually discharged if there are no notable problems after surgery, there may be many cases where hyperthyroidism is overlooked if the problem is not immediately discovered. Therefore, if a repeated peptic ulcer is accompanied by hyperbilirubinemia, we cautiously suggest considering hyperthyroidism as the cause. This case has some significance in that it proposes a hypothesis that there is a correlation between hyperthyroidism and peptic ulcer disease. A prospective case-control study on this topic is warranted in the future.
Acknowledgments
We would like to thank the patient who allowed us to present the case. And I would like to express my gratitude to everyone in the emergency intensive care unit who helped facilitate the treatment.
Notes
Author Contributions
Conceptualization: SHL, Methodology: SHL, JYJ. Formal investigation: SHL, JYJ, KHP. Writing original draft: KHP, SHL, Writing - review and editing: SHL, JYJ.
Figure 1
Abdominopelvic computed tomography scan at the time of admission. (A) Perforation site of the duodenal first portion with air bubbles (white arrow). (B) Fluid collection at periperforation site and free air (white arrow).

Figure 2
Computed tomography angiography to determine the cause of hyperbilirubinemia. (A) Linear high attenuation embolic materials along right hepatic artery (white arrow). (B) Linear high attenuation embolic materials along left hepatic artery (white arrow). (C) Embolic materials along left hepatic artery in the axial view (white arrow).
References
1. Townsend CM Jr, Counteny M, Beauchamp RD, Evers BM, Mattox KL. editors. Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. 21st ed. Townsend Courtney. Philadelphia (PA): Elsevier Saunders; 2021.
2. Steinheber FU. Medical conditions mimicking the acute surgical abdomen. Med Clin North Am 1973;57(6):1559–67.


3. Leow MK, Chew DE, Zhu M, Soon PC. Thyrotoxicosis and acute abdomen--still as defying and misunderstood today? Brief observations over the recent decade. QJM 2008;101(12):943–7.


4. Aoyagi K, Sengoku K, Hatano M, Hamaguchi E, Shimomura T. Relationship between hyperthyroidism and peptic ulcer. Dtsch Z Verdau Stoffwechselkr 1973;33(6):281–91. [in German].

6. Garbat AL. The simultaneous occurrence of active peptic ulcer and active hyperthyroidism. J Mt Sinai Hosp N Y 1951;17(6):787–92.

7. Natsuda S, Nakashima Y, Horie I, Ando T, Kawakami A. Thyroid Storm Precipitated by Duodenal Ulcer Perforation. Case Rep Endocrinol 2015;2015:750390.



10. Seino Y, Matsukura S, Miyamoto Y, Goto Y, Taminato T, Imura H. Hypergastrinemia in hyperthyroidism. J Clin Endocrinol Metab 1976;43(4):852–5.


11. Koyuncu A, Aydintu S, Koçak S, Aydin C, Demirer S, Topçu O, et al. Effect of thyroid hormones on stress ulcer formation. ANZ J Surg 2002;72(9):672–5.


13. Park JT, Lim HK, Park JH, Lee KH. Thyroid storm during induction of anesthesia. Korean J Anesthesiol 2012;63(5):477–8.



14. Piantanida E. Preoperative management in patients with Graves’ disease. Gland Surg 2017;6(5):476–81.



15. Palace MR. Perioperative Management of Thyroid Dysfunction. Health services insights 2017;10:1178632916689677.



17. Myers JD, Brannon ES, Holland BC. A correlative study of the cardiac output and the hepatic circulation in hyperthyroidism. J Clin Invest 1950;29(8):1069–77.