Acute Kidney Injury After Trauma: Risk Factors and Clinical Outcomes
Article information
Abstract
Purpose
Acute kidney injury (AKI) is an uncommon but serious complication after trauma. The objective of this study was to evaluate the clinical characteristics, risk factors, and outcomes of AKI after trauma.
Methods
A retrospective cohort study of 386 trauma patients who visited the emergency department at the Asan Medical Center between January 2012 and December 2013 was performed. There were 322 patients included in this study. Patients were assigned into the AKI group and no AKI group. Regression analysis was performed to identify the factors associated with development of AKI following trauma.
Results
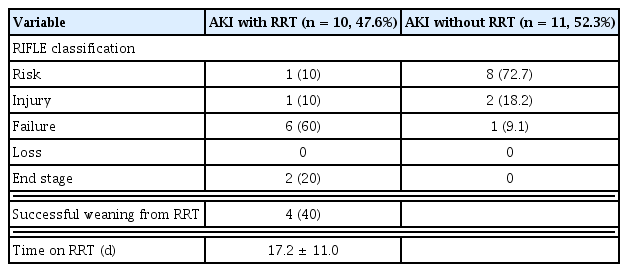
The overall incidence of AKI following trauma was 6%. There was no difference in patients’ age, sex, and body weight between groups. Whereas there was a significant difference in Injury Severity Score, Glasgow Coma Scale, presence of shock, need for a transfusion, lactic acid levels, and severe rhabdomyolysis. In multivariate analysis, the independent risk factors associated with AKI after trauma included the Injury Severity Score [odds ratio (OR) = 1.065, p < 0.01], presence of shock (OR = 3.949, p = 0.012), and severe rhabdomyolysis (OR = 4.475, p < 0.01). Patients in the AKI group were classified (according to the RIFLE criteria) as at Risk in 9 cases (43%), Injury present in 3 (14%), Failure in 7 (33%), Loss in 0 (0%) and End-stage in 2 (10%). Renal replacement therapy was required for 10 patients (47%) in the AKI group and 4 of them (40%) underwent successful weaning. Hospital mortality rate was higher in the AKI group (5/21, 23%) than the no AKI group (3/301, 1%; p < 0.01).
Conclusion
The development of AKI was associated with the severity of trauma, and trauma increased mortality rates.
Introduction
Acute kidney injury (AKI) can occur in trauma patients due to direct damage to the kidney or indirect damage caused by hemorrhagic and septic shock [1,2]. Though the incidence of acute kidney injury in trauma patients is only 15%, such injuries often occur in critically ill patients who require intensive care [3]. Some patients with AKI can recover renal function with conservative treatment. However, trauma patients who have AKI which requires renal replacement therapy have a high mortality rate (29.6%) during hospitalization. Patients who do not recover from AKI and progress to end-stage renal disease require constant renal replacement therapy, which increases patient morbidity and mortality. Furthermore, as the associated cost would be very high, prompt treatment at the early stages is clinically important [4,5]. This study was performed to evaluate the clinical characteristics of patients with AKI resulting from trauma. The risk factors were examined, the progression of treatment was evaluated, and the effects of AKI caused by trauma were analyzed.
Materials and Methods
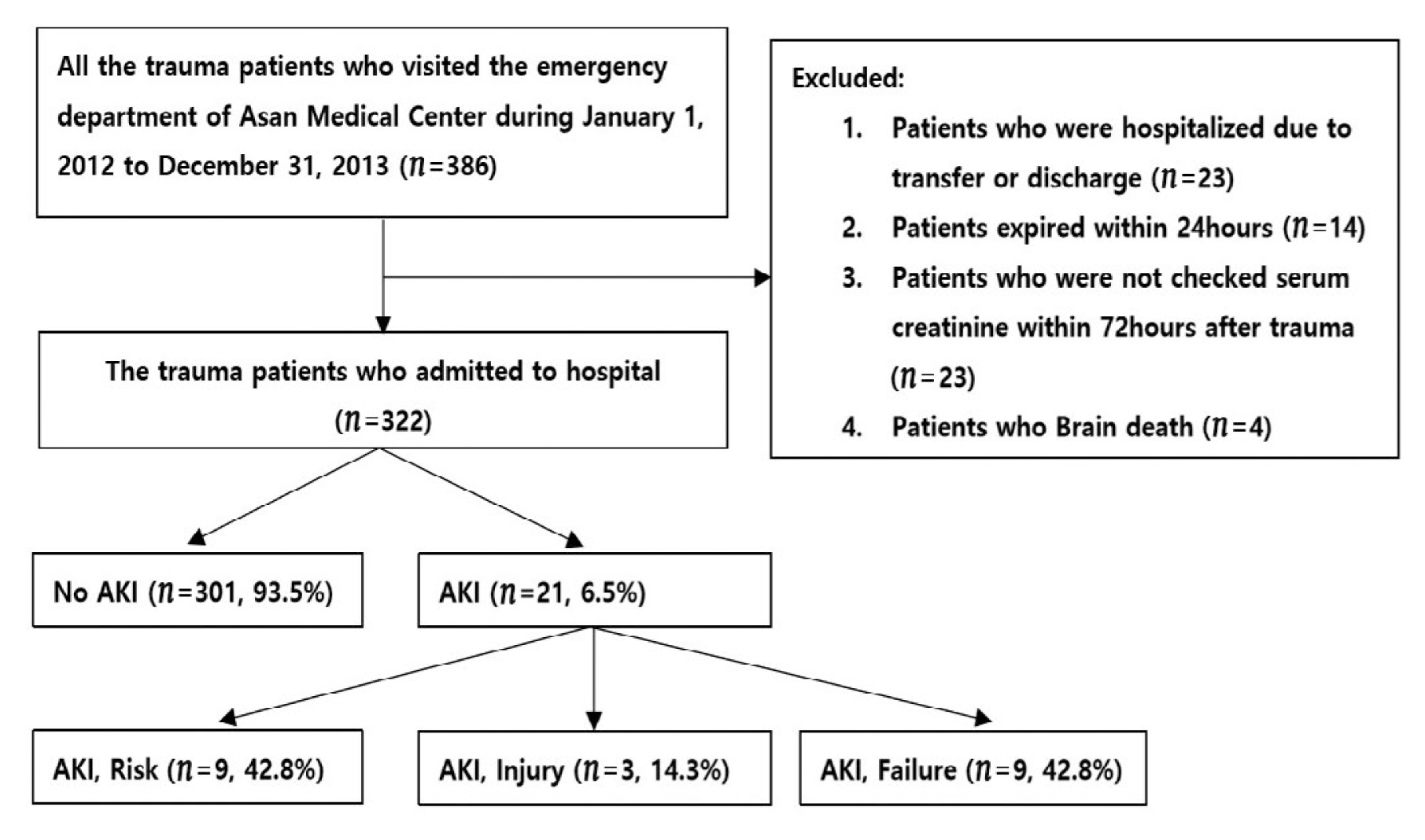
Data for trauma patients who visited the emergency room at the Asan medical center from January 1st, 2012 to December 31st, 2013 were retrospectively analyzed. From 386 patients who visited the center, 23 patients were excluded from the study because they left the hospital on the same day or were transferred to a different hospital, 14 patients died within 24 hours, 23 patients visited the center 72 hours after they were injured, and 4 were brain-dead patients. The remaining 322 patients were included in this study. Gender, age, underlying disease, duration of hospital stay, and survival status of the patients were retrieved from patient medical records. To determine the effects of trauma the Injury Severity Score (ISS) and, the Glasgow Coma Scale (GCS) were administered at admission, and the presence of hypotensive shock (systolic blood pressure below 90 mmHg) was recorded. Blood transfusion status and the amount of packed red blood cells in the transfusion were recorded. Blood urea nitrogen, serum creatinine, and serum creatinine kinase (CK) concentrations in the blood were assessed, and the base excess and lactate levels through arterial blood gas analysis were checked.
In this study, patients who underwent computed tomography (CT) scans within 72 hours of visiting the hospital were examined and it was noted whether contrast media was used. The process for patients with AKI who underwent renal replacement therapy was recorded. The number of days spent in the intensive care unit, hospital length of stay, ventilator period, and mortality in patients with and without AKI were also compared and analyzed. An increase in serum creatinine levels to 1.5 times the level recorded at hospital admission, taken within 72 hours, was defined as AKI. Among the 322 patients in the study, 21 patients met this criterion. Based on the RIFLE diagnostic criteria, patients with more than a 1.5-fold increase in serum creatinine level were classified into the Risk group, those with more than a 2-fold increase were placed into the Injury group, and those with more than a 3-fold increase or without urine production were assigned to the Failure group (Figure 1) [6]. If the level of CK was above 5,000 IU/L, within 72 hours after hospital admission, severe rhabdomyolysis was diagnosed [7]. SPSS Version 18.0.0 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. Analysis of variance and the t test were used to determine statistical differences between populations. Chi-squared test was used to determine whether there were associations between variables and odds ratio (OR) was used to determine the strength of association of risk using cross analysis through binary logistic regression.
Results
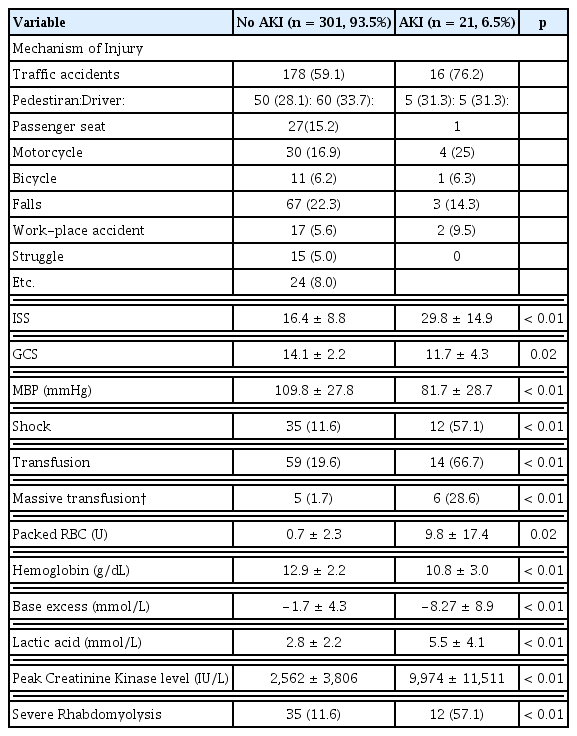
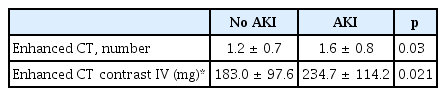
The average age of the patients included in this study was 48.9 ± 18.6 years. There were 234 men (72.7%) and 88 women (27.3%), and AKI occurred in 21 patients (6.5%). There was no difference in the average age of patients that experienced AKI and those who did not (p = 0.09), nor was there a significant difference in gender distribution (p = 0.378). The underlying conditions including hypertension (p = 0.644), diabetes (p = 0.640), and history of a stroke (p = 0.712) did not show significant differences between the 2 groups (Table 1). Traffic accidents were the most common causes of trauma in the 2 groups, followed by injuries caused by a fall. The ISS was higher for the patients that suffered AKI, (16.4 ± 8.8 vs. 29.8 ± 14.9, p < 0.01) and the GCS value was lower (14.1 ± 2.2 vs. 11.7 ± 4.3, p = 0.02). The frequency of hypotensive shock was also higher in the AKI group (11.6% vs. 57%, p < 0.01) and the level of hemoglobin in the serum test which was performed at hospital admission was lower (12.9 ± 2.2 vs. 10.8 ± 3.0, p < 0.01). Patients with AKI required more blood transfusions (19.6% vs. 66%, p < 0.01) and the amount of packed red blood cells in the transfusion was higher (0.7 ± 2.3 vs. 9.8 ± 17.4, p = 0.02). Acid-base imbalance was greater in these AKI patients (−1.7 ± 4.3 vs. −8.27 ± 8.9, p < 0.01) as well as the serum lactate concentration (2.8 ± 2.2 vs. 5.5 ± 4.1, p = 0.01). The serum CK level was higher in the patients with AKI (2,562 ± 3,806 vs. 9,974 ± 11,511, p < 0.01), and the frequency of severe rhabdomyolysis was higher (11.6% vs. 57.1%, p < 0.01; Table 2). The amount of contrast media (Xenetix350: iobitridol/150 mg) used for CT scans was also higher for patients with AKI (p < 0.02; Table 3).
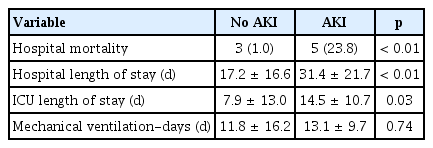
Univariate logistic regression, showed that trauma patients with AKI had statistically significant differences in ISS, GCS, blood pressure, shock, transfusion, hemoglobin, acid-base balance, lactate concentration, CK level, and severe rhabdomyolysis (p < 0.01). After adjusting for confounders, the occurrence of AKI was investigated as an independent variable. A higher frequency of AKI was observed when the ISS was high (OR 1.06, p < 0.01), when the patient had hypotensive shock at the time of trauma (OR 3.949, p = 0.01), and when severe rhabdomyolysis occurred (OR 4.475, p < 0.01). Transfusion, lactate concentration, and acid-base imbalance were not identified as independent variables (Table 4). There were 10 (47%) of the 21 patients with AKI who received renal replacement therapy: 1 patient in the Risk group, 1 in the Injury group, and 8 in the Failure group. The patients with AKI had a longer length of stay in hospital (31.4 ± 21.7 vs. 17.2 ± 16.6, p < 0.01), a longer intensive care period (14.5 ± 10.7 vs. 7.9 ± 13.0, p = 0.03), and higher mortality (5% vs. 3%, p < 0.01; Table 5). There were 4 out of the 10 patients who received renal replacement therapy who eventually recovered renal function, and dialysis treatment was discontinued. There were 2 patients who required ongoing dialysis treatment throughout the observation period of 6 months and 4 died during treatment (Figure 2, Table 6).
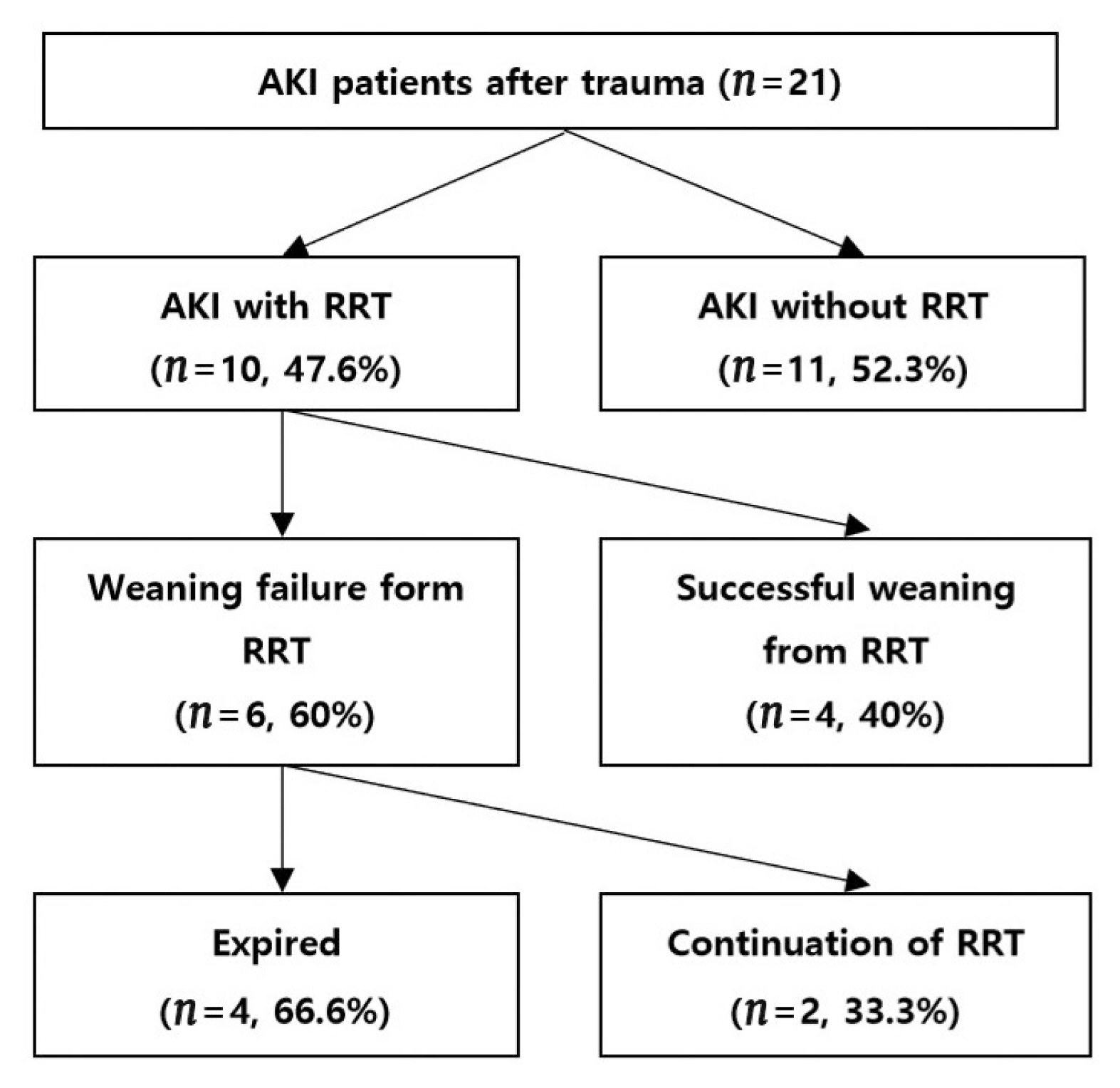
Clinical manifestation of AKI patients after trauma over 6 months.
AKI = acute kidney injury; RRT = renal replacement therapy.
Discussion
The reported prevalence of AKI in trauma patients is 8.4–10.2% [7,8]. Additionally, 1.5–24% of AKI occur in chronic patients who receive treatment in the intensive care unit [9]. AKI has been reported in 30% of patients who experienced major trauma [2,10]. In the current study, AKI was observed in 21 (6.5%) out of 322 trauma patients, accounting for 12.1% of 174 patients who received intensive care.
AKI in trauma patients is usually attributed to direct injuries to the kidney or rhabdomyolysis, which occurs when substances from cell injury circulate within the body. AKI, following a decline in renal perfusion due to bleeding, shock, and blood poisoning has also been reported [11]. AKI due to rhabdomyolysis occurs because muscle enzymes released into circulation from the sarcolemma (following damage to skeletal muscle tissues) degrade the function of glomerular cells in the kidney, and cause the glomerular filtration rate to decrease. In addition, the disappearance of adenosine triphosphate in monocytes and the increase of calcium in cells cause renal cell injury [12]. It has been reported that AKI can occur in 13% to 50% of trauma patients with rhabdomyolysis and cause a mortality rate of 17.5–40% [13,14]. In this study, rhabdomyolysis occurred in 23% of patients. Among patients with severe rhabdomyolysis, the occurrence of AKI as an independent variable was 4.48 times higher.
The ISS indicates the degree of injury in trauma patients and is correlated with the occurrence of AKI [15,16]. The ISS for trauma patients at hospital admission was observed to be an independent variable, and a 1-point increase in the ISS corresponded to an increase in the risk of AKI by 1.07 times. Bleeding following trauma can cause hypotensive shock which deteriorates the function of the kidneys, and causes AKI by inducing cell hypoxia and renal ischemia following low perfusion in the renal vascular system [9]. Eriksson et al [17] reported that massive bleeding was a yardstick for AKI. In their study, the occurrence of AKI was 3.95 times higher in patients that suffered hypotensive shock. However, factors that affect the degree of hypotensive shock, such as total transfusion, lactic acid, acid-base balance in the body, and hemoglobin level were not correlated with the occurrence of AKI. The contrast medium, iobitridol is absorbed in the body and increases the accuracy of observation in the area of acute hemorrhage during a CT scan. However, it has been reported that iobitridol can induce external acute rejection reactions and malfunction in renal cells [18]. In this current study the correlation between the use of contrast media for trauma patients was examined between groups with and without AKI, and it was determined that contrast media was used more frequently for the group without AKI but this observation was not statistically significant (p = 0.03). Despite the higher number of examinations due to multiple injuries, leading to the application of more contrast media, the amount of contrast media administered was not a direct independent variable for the occurrence of AKI.
Patients with AKI may undergo renal replacement therapy according to the degree of kidney function degradation to prevent complications due to acid-base and electrolyte imbalance [2]. In this current study, 10 (47%) of 21 patients with AKI received renal replacement therapy for an average of 17 days. There were 4 (40%) of these patients who recovered kidney function and discontinued renal replacement therapy, 2 (20%) progressed to chronic renal injury and continued renal replacement therapy, and 4 patients (40%) died. The hospitalization and treatment periods for AKI patients in critical condition were longer. The mortality rate among patients with normal renal function was 1% whereas patients with AKI had a high mortality of 23%. In consideration of the 17–43% mortality reported in other studies [19,20], future studies are warranted to assess the occurrence of AKI in trauma patients, understand the degree of injury, and set up a systematic treatment plan. This is a retrospective study and thus, various biases were inevitable. As this study was performed in only 1 hospital, there may be differences in the rate and seriousness of injuries. Another limitation is that 6 months of observation is a little short. Though we reported the risk factors for the occurrence of trauma and AKI, more studies are needed to determine the relationship between trauma and AKI.
Conclusion
AKI in trauma patients extends the recovery period and increases mortality. Therefore, clinicians need to determine the degree of trauma in the early stages, maintain perfusion, administer treatment for rhabdomyolysis, and reduce the incidence of AKI.
Notes
Conflicts of Interest
The authors have no conflicts of interest to declare.