Feasibility of Peripherally Inserted Central Catheter Placement in COVID-19 Patients Isolated in the Intensive Care Unit of a Small Volume Center (291-Bed Hospital)
Article information
Abstract
Purpose
Patients with coronavirus disease 2019 (COVID-19) should be isolated from others to prevent widespread infection. The purpose of this study was to evaluate the feasibility of performing peripherally inserted central catheter (PICC) placement in patients with COVID-19 isolated in the intensive care unit (ICU) of a small volume center hospital.
Methods
This retrospective study included 79 patients who underwent PICC in 2 ICUs. There were 41 patients with COVID-19 who were isolated in an ICU (isolated ICU) and there were 38 patients who required ICU care who did not have COVID-19 (non-isolated ICU). Their medical records including PICC-related complications and clinical variables were compared.
Results
PICC placement was performed to maintain long-term intravenous access for 78% of the COVID-19 group and 52.6% of the non-COVID-19 group (p = 0.017). The mean procedure time (minutes) was 15.2 ± 7.58 in the COVID-19 group and 12.6 ± 6.65 in the non-COVID-19 group (p = 0.109). When PICC tip locations were divided into three groups (optimal, suboptimal, and malpositioned), there was no significant difference between the two groups of patients. PICC-related complications in COVID-19 and non-COVID-19 groups included non-functioning catheter (0% vs. 5.3%, p = 0.137), occurrence of swelling or hematoma around PICC inserted site (2.4% vs. 0%, p = 0.333), and PICC-related infection.
Conclusion
PICC placement for patients with COVID-19 isolated in the ICU of a small volume center hospital was feasible and safe.
Introduction
The coronavirus disease 2019 (COVID-19) outbreak started in 2019 [1]. In South Korea, hospitals of various grades have been dedicated to treatment of patients with COVID-19 in response to the rapid increase in the numbers of the population becoming infected. General hospitals in South Korea as defined by law are equipped with over 100 beds and large volume centers have more than 300 beds [2]. However, hospitals with more than 500 beds have been usually referred to as large volume centers. In this study, general hospitals with 100 to 300 beds were defined as small volume centers.
During the COVID-19 pandemic, medical staff complied with Korean Centers for Disease Control and Prevention guidelines for the treatment of patients with COVID-19. They wore Level D coveralls [as personal protective equipment (PPE)] [3] overlaid with a sterile gown and gloves when they performed procedures for COVID-19 patients in an isolated space (Figure 1). Under these conditions it was difficult to perform procedures. Intensivists working in the intensive care unit (ICU) of small volume centers occasionally experienced situations where there was a lack of interventionists and vascular surgeons. Therefore, due to the lack of support in a situation that required the wearing of PPE which was uncomfortable, it was more difficult to perform procedures.
Operator was dressed in Level D coverall with a face shield, sterile gown, and gloves during preparation of PICC placement.
PICC = peripherally inserted central catheter.
Peripherally inserted central catheters (PICCs) have been widely used as an alternative to traditional central catheters and are associated with a relatively low incidence of complications [4–6]. PICCs are usually performed in an intervention or operation room with fluoroscopic guidance [7,8]. However, transporting patients in the ICU to other spaces is difficult because these patients require specific management such as various monitoring systems, continuous drug administration, and ventilator support [9,10]. Although previous studies have reported that performing placement of PICCs at a patient’s bedside is feasible and safe [11–13], PICCs are usually performed in large volume centers in a situation without restrictions on the number of medical staff or with the level of PPE required on a COVID-19 isolated ICU. The aim of this study was to evaluate the feasibility of performing PICC placement in patients with COVID-19 isolated in the ICU of a small volume center.
Materials and Methods
This retrospective study was approved by the institutional review board (IRB) of H plus Yangji hospital (IRB no: HYJ 2022-07-018) and adhered to the tenets of the Declaration of Helsinki. This study was performed based on medical records of a total of 79 patients who underwent PICC in H plus Yangji Hospital (291-beds), a secondary referral center in Seoul, Korea. Part of this hospital has been dedicated to COVID-19. In the hospital there is a 13-bed ICU and there is also an 8-bed ICU reserved for patients with COVID-19. These two ICUs are separate.
PICC placement was performed at the bedside for patients with COVID-19 because intervention and operation rooms were not dedicated to COVID-19. For patients without COVID-19, PICC placement was either performed at the patient’s bedside or in the intervention room depending on the situation.
All procedures were performed by a single intensivist using, a scale-5 French Dual Lumen Power PICC Catheter (Bard Access System, Inc., Utah, USA). The patient was in a supine position with arms abducted. Tourniquets were applied around patient’s upper arm. The procedure was performed under maximal barrier precautions including skin preparation with 2% chlorhexidine. The patient was covered with surgical drapes. The operator was dressed in a Level D coverall overlaid with a sterile gown and gloves and the procedure was performed in the isolated ICU. In non-isolated ICU, the operator was dressed in a surgical cap, a sterile gown, and gloves.
PICC placement was performed in three steps. In the 1st step, an appropriate puncture into the target vein was performed. In the 2nd step, the catheter was advanced from the insertion site to around the optimal position. In the 3rd step, the optimal position was located. In this study, the 1st step began with selecting a vein which was visible and easy to puncture. Typically, a basilic or brachial vein was selected. Puncture into a cephalic vein is relatively difficult as it is a superficial vein that is usually smaller. In case of failure to puncture in the 1st attempt, a previous puncture site should be compressed adequately. Otherwise, this may hinder the 2nd attempt puncture due to hematoma from injured (puncture) tissues such as vein, artery, and muscle. Procedures were performed with pressure-free handling of an ultrasound probe because the target vein was already compressed by the probe. When the tip of the needle used to puncture the vein was not located in the middle of the lumen of the target vein, the guidewire might not be able to be advanced with enough length, although blood from the target vein maybe flowing out through the puncture. In addition, detecting the catheter tip could be difficult because the face shield and light in the room could blur the operator’s visual field whilst watching the ultrasound monitor. In addition, the light in the room might reflect on the ultrasound monitor. The 2nd step was to advance the guidewire with enough length so that it located above the axillary area. After the guidewire had been inserted with enough length, catheter insertion was then performed. When the guidewire had not advanced above the axillary area, another attempt was made after removing the guidewire. In the 1st attempt to insert the catheter, the guidewire was removed after an introducer was inserted. Catheter insertion was then tried. When the catheter could not be advanced, another attempt was performed. To avoid vessel injury careful handling was required with the catheter and guidewire, and monitoring for the detection of guidewire induced arrhythmia was performed. The 3rd step was to locate the catheter optimally. The catheter length was measured by adding the distance from the insertion site to the axilla, axilla to sternum, and to the 4th intercostal space [12]. The catheter tip position could be categorized into three groups; optimal, suboptimal, and malposition. The optimal position was defined as a catheter at the point of the cavoatrial junction and approval range was set up from the distal two thirds of superior vena cava (SVC) to upper atrium (RA; Figure 2A). The suboptimal position was defined as a catheter within the RA or SVC tributary. Malposition was defined as a catheter tip in other veins. After catheter insertion was completed, the internal jugular vein (IJV) was scanned under ultrasound to assess catheter placement. It should be noted that it was difficult to judge whether the catheter tip was located in a non-optimal position, (suboptimal and malposition) except IJV, without fluoroscopic guidance. When the catheter length was too short or too long to be placed in an optimal position, the tips of the catheters were located in the SCV, SVC tributary, and RA. In addition, it was difficult to determine whether the catheter tip was in the vein of the arm. For instance, if the catheter was advanced from the brachial vein to the cephalic vein. Therefore, a chest X ray was performed to verify the location of the catheter tip after all procedures had been performed. Suboptimal positioned catheters were maintained whereas malpositioned catheters were changed in this study.
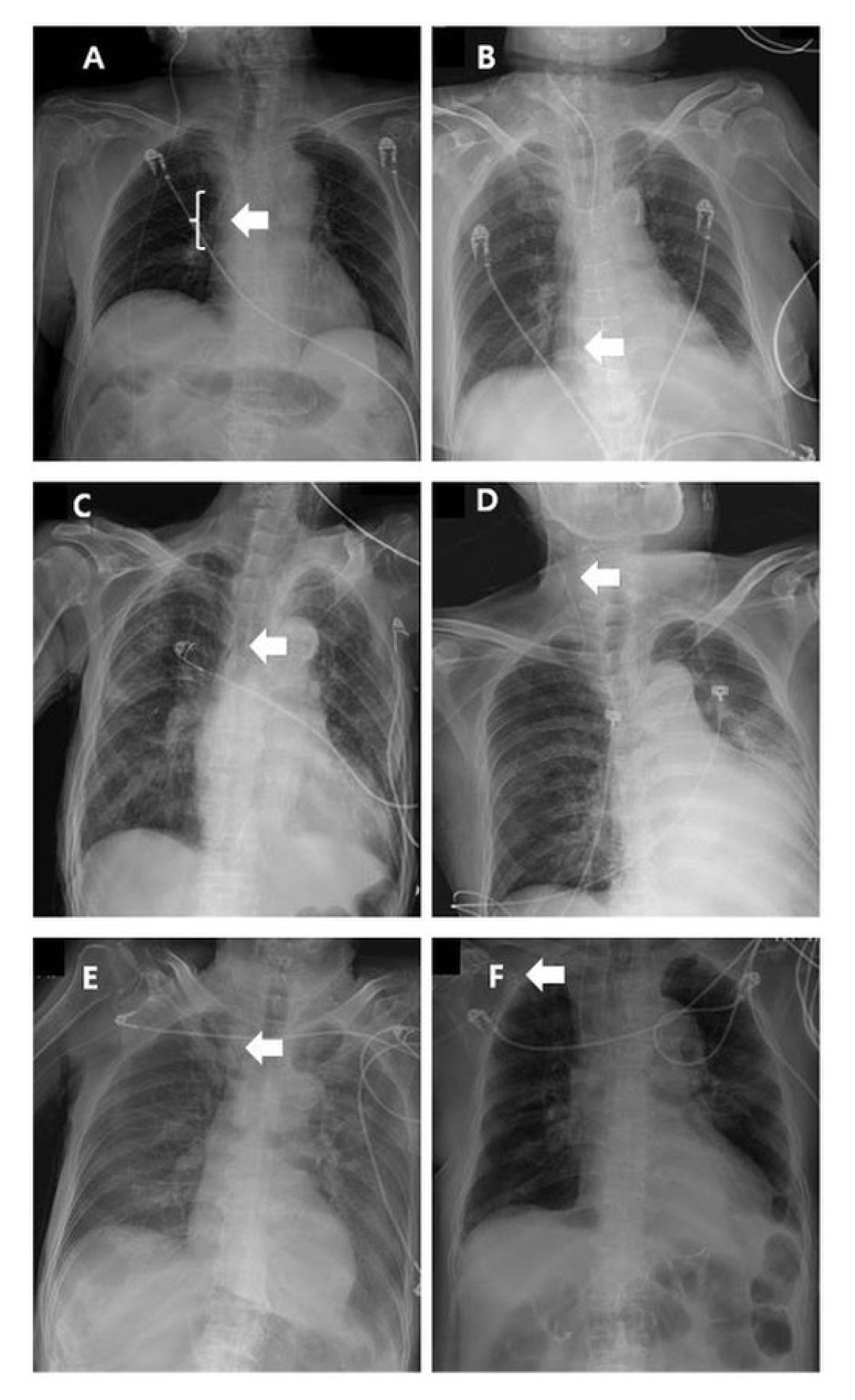
PICC tip locations. (A) Optimal position. PICC tip in atriocaval junction (range of optimal position was defined from distal 2/3 of SVC to upper RA); (B and C). Suboptimal positions. PICC tips in RA and SVC tributary; (D, E, and F)
Malpositions. PICC tips in IJV, SCV, and the other arm vein.
The white arrow indicates the PICC tip.
IJV = internal jugular vein; PICC = peripherally inserted central catheter; RA = right atrium; SCV = subclavian vein; SVC = superior vena cava.
Continuous variables were analyzed using the student t test. Categorical variables were analyzed using the chi-square test. A p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics Version 28.0.1.1. (IBM, Amonk, NY, USA).
Results
1. Clinical characteristics of the study population
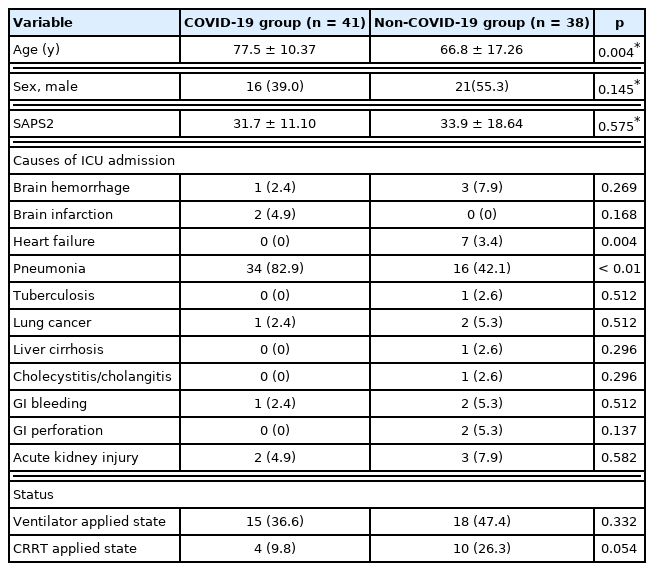
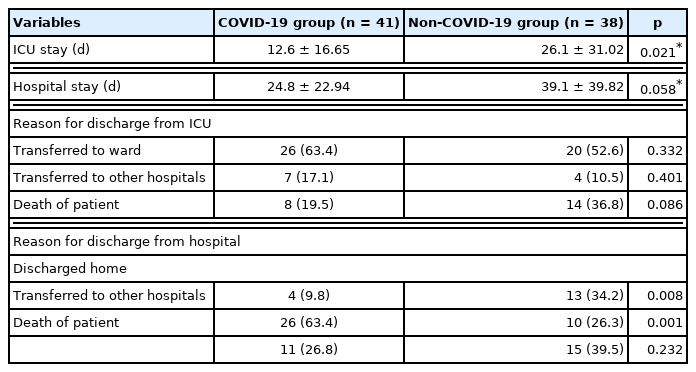
In this study, a total of 79 patients underwent PICC placement in 1 of the 2 ICUs of the hospital. Patients with COVID-19 (n = 41, 51.9%) were in the isolated ICU and patients without COVID-19 (n = 38, 48.1%) were in the non-isolated ICU (Tables 1 and 2). The mean age was significantly higher in the COVID-19 group compared with the non-COVID-19 group (77.5 ± 10.37 years vs. 66.8 ± 17.26 years, p = 0.004). Males accounted for 39.0% (n = 16) in the COVID-19 group and 55.3% (n = 21) in the non-COVID-19 group (p = 0.145). The Simplified Acute Physiologic Score 2 was 31.7 ± 11.10 in the COVID-19 group and 33.9 ± 18.64 (p = 0.575) in the non-COVID-19 group. The proportion of patients with heart failure as the cause of ICU admission showed a significant difference between the two groups (0%, n = 0 vs. 3.4%, n = 7, p = 0.004). The proportion of patients requiring a ventilator showed no significant difference between the two groups (36.6%, n = 15 vs. 47.4%, n = 18, p = 0.332). The proportion of patients with continuous renal replacement therapy was similar between the two groups (9.8%, n = 4 vs. 26.3%, n = 10, p = 0.054). and the total length of stay in ICU was 12.6 ± 16.65 days in the COVID-19 groups and 26.1 ± 31.02 days (p = 0.021) in the non-COVID-19. The total length of stay in hospital was 24.8 ± 22.94 days in the COVID-19 group and 39.1 ± 39.82 days in the non- COVID-19 group (p = 0.058). The reasons for discharge from the ICU were divided into three categories: (1) patients who were transferred to the general ward; (2) patients who were transferred to other hospitals; and (3) death of the patients. These three categories showed significant differences between COVID-19 and non-COVID-19 (1st category: 63.4%, n = 26 vs. 52.6%, n = 20, p = 0.332; 2nd category: 17.1%, n = 7 vs. 10.5%, n = 4, p = 0.401; and 3rd category: 19.5%, n = 8 vs. 36.8, n = 14, p = 0.086). Reasons for discharge from hospital were also divided into three categories: (1) discharged home; (2) discharged to other hospitals; and (3) death of the patients. The proportions of patients in these three categories were 9.8% (n = 4), 63.4% (n = 26), and 26.8% (n = 11) in the COVID-19 group and 34.2% (n = 13), 26.3% (n = 10), and 39.5% (n = 15) in the non-COVID-19 group (p = 0.008, p = 0.001, and p = 0.232, respectively).
2. PICC-related variables
PICC-related variables are shown in Table 3. The purpose of PICC placement could be divided into two categories. The 1st was for maintaining long-term intravenous (IV) access with 78.0% (32/41) in the COVID-19 group vs. 52.6% (20/38) in the non-COVID-19 group, p = 0.017). The 2nd was for using nonperipherally compatible infusate. The non-COVID-19 group had more patients with previous catheterization (34.2%, n = 13) than the COVID-19 group (9.3%, n = 5; p = 0.020). In the COVID-19 group, the catheter was inserted in the right and left arms 63.4% and 36.6% had a mean catheter length of 38.5 ± 2.35 cm and 43.9 ± 3.43 cm, respectively). In the non-COVID-19 group, these numbers were 52.6% and 47.4% with mean catheter length of 39.4 ± 2.87 cm and 45.9 ± 2.90 cm, respectively. Accessed veins included the brachial, basilic, and cephalic veins (61.0%, 34.1%, and 4.9% in the COVID-19 group; 63.2%, 28.9%, and 7.9% in the non-COVID-19 group; p = 0.842, p = 0.620, and p = 0.582, respectively). The mean procedure time was 15.2 ± 7.58 minutes in the COVID-19 group and 12.6 ± 6.65 minutes in the non-COVID-19 group (p = 1.109). The mean indwelling time was 12.7 ± 8.26 days in the COVID-19 group and 15.3 ± 9.97 days in the COVID-19 group (p = 2.204).
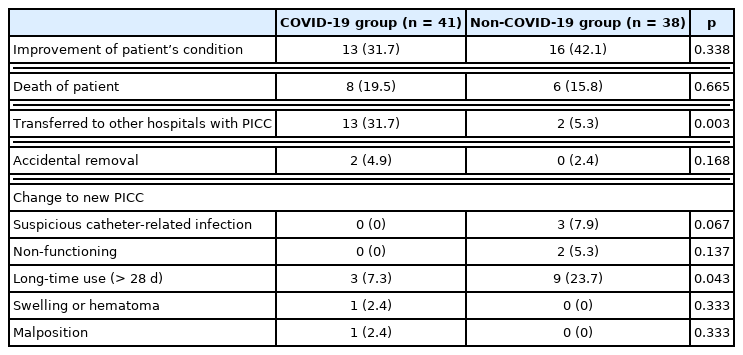
The reasons for PICC removal include the following: (1) improvement in the patient’s condition (31.7% vs. 42.1%, p = 0.338); (2) death of patient (19.5% vs. 15.8%, p = 0.665); (3) transferred to other hospitals with PICC (31.7% vs. 5.3%, p = 0.003); (4) accidental removal (4.9% vs. 0%, p = 0.16); and (5) change to new PICC (Table 4). Catheters were changed due to suspicion of catheter-related infection (0% vs. 7.9%, p = 0.067), non-functioning catheter (0% vs 5.3%, p = 0.137), long-time use i.e., > 28 days (7.3% vs. 23.7%, p = 0.043), the occurrence of a swelling or hematoma around the catheter inserted site (2.4% vs. 0%, p = 0.333), and malposition (2.4% vs. 0%, p = 0.333). More patients were transferred to other hospitals with PICC in the COVID-19 group compared with patients who were in ICU with no COVID-19 (p = 0.003).
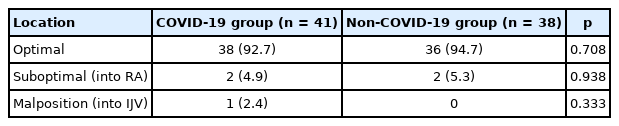
Catheter tip locations were divided into three categories: optimal, suboptimal including the catheter tip in the RA and SVC tributary (Figures 2B and C), and malposition (92.7%, 4.9%, and 2.4% in the COVID-19 group vs. 94.7%, 5.3%, and 0% in the non-COVID-19 group, p = 0.708, p = 0.938, and p = 0.333, respectively; Table 5). In this study, there was one case of a malpositioned catheter tip placed into the IJV (Figure 2D). However, there was no case of catheter tip in the SVC tributary, SCV, or arm vein in this study. Referentially, cases shown in Figures 2C, E, and F were not included in this study because while the same operator performed the PICC placements, they were performed outside of the study period. Catheter-related complications included non-functioning catheter, occurrence of a swelling or hematoma around catheter inserted site, and a catheter-related infection. Culture results, revealed Sternotrophomonas mlatophilia as the pathogenic agent for one case in the non-COVID-19 group (Table 6).
Discussion
In South Korea, outbreaks of serious infectious diseases have occurred throughout the country several times, such as, severe acute respiratory syndrome [14], novel swine-origin influenza A [15], Middle East respiratory syndrome [16,17], and COVID-19. When the number of patients with COVID-19 rapidly increased, the medical system in the whole country had to respond to the widespread infection. Several large volume centers, public hospitals, and other general hospitals have afforded medical services including hospitalization for the isolation of patients. Medical staff dressed in PPE cared for these patients. The workload was more difficult because PPE is uncomfortable to work in. Staff in PPE had to work in sweltering heat. Additionally, time out of the shift was needed for dressing and undressing. Furthermore, medical staff dressed PPE had prolonged shifts in the isolated ICU and frequently entered. Moreover, the number of bedside procedures increased because of restricted patient transport. Staff fatigue under these situations may have resulted from the relative lack of staff on a shift for ICUs in small volume centers. Therefore, whether basic procedures such as PICC placement could be performed without effort or not should be accessed.
This study compared the outcomes of patients who underwent PICC placement in the isolated ICU where the operator had to wear PPE and in the non-isolated ICU where patients underwent PICC placement by an operator without wearing the same level of PPE required to work in the isolated ICU.
In this study, there were more patients who underwent PICC placement for long-term maintenance of IV access in the COVID-19 group. It could be assumed that ICU nurses wearing PPE had more difficulty to access peripheral IV placement. In addition, for patients with COVID-19 in the restricted area there was difficulty changing peripheral IV access frequently. On the other hand, there were more patients who underwent PICC placement after previous catheterizations such as CVC and PICC in the non-COVID-19 group. Thus, PICC might be more favorable for easy management and maintenance of IV access compared with peripheral IV access in an uncomfortable situation.
Previous studies have reported that a non-optimal position of catheter tip could induce complications such as occlusion, non-function, arrhythmia, and thrombosis [18–21]. The location of an optimal PICC tip has not been defined clearly [22]. In this study, the range of the optimal position was defined from the distal two thirds of SVC to the upper RA, which has been generally acceptable [23].
Catheter-related complications showed no significant differences between the two groups. However, the actual catheter-related infection rate could be higher because catheter tip culture from patients who expired following the transfer to another hospital was not performed.
This study has several limitations. Firstly, data were analyzed retrospectively. In addition, the amount of the data was small. Further studies are needed to determine late complications such as catheter-related infections.
In conclusion, analyses of PICC-related outcome in this study may indicate that the required level of PPE resulting in staff feeling uncomfortable and the lack of manpower should be less restrictive when doing the PICC placement. Therefore, there is reasonable indication that it is feasible and safe to perform PICC placement for isolated patient with COVID-19 (or isolated due to other infectious diseases) in small volume center ICUs.
Notes
Author Contributions
Conceptualization: Formal analysis: KWL. Data analysis: KWL. Writing original draft: KWL. Writing-review and editing: MCK and KWL.
Conflicts of Interest
The authors have no conflicts of interest to declare
Ethical Statement
This study was approved by the IRB of H plus Yangji Hospital (IRB no.: HYJ 2022-07-018) and adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived by IRB of H plus Yangji Hospital because of the retrospective nature of this study. All personal identification of the patients was removed.
Data Availability
All relevant data are included in this manuscript.
Funding
None.