Let’s Get That Bread Clip: Mechanical or Malignant Large Bowel Obstruction?
Article information
Abstract
A 60-year-old female presented with symptoms consistent with a large bowel obstruction (LBO). Following confirmation of LBO using imaging, she progressed to a laparotomy which potentially revealed a large rectosigmoid tumor with surrounding adhesions, deemed unresectable. The postoperative course was complicated by an enterocutaneous fistula. She was transferred to a tertiary center and underwent a repeat laparotomy which revealed a large fibrotic mass associated with an intra-luminal bread clip (expiry date 2002). This case report details the interesting causative nature of this LBO and the subsequent surgical management, and complicated postoperative course.
Introduction
Bowel obstructions are a frequent cause of presentation at hospital, and 20% to 25% of these obstructions are in the large bowel [1]. Patients present with symptoms such as nausea, abdominal distension, obstipation, and generalized cramping pain. Vomiting is considered to be a late sign of obstruction [2].
This report presents a large bowel obstruction (LBO) which was initially managed as a malignant etiology. This management was then prolonged by several postoperative complications requiring transfer from a rural to a metropolitan center for specialist colorectal management. It was identified that the patient’s symptoms were not caused by an obstructing rectosigmoid lesion, but by an abscess formed around a bread clip that appeared to have been ingested 20 years prior.
This case highlights some of the key discussion on the management of a suspected malignant LBO and the different operative strategies that can be employed.
Case Report
A 60-year-old female presented with 2 weeks of central abdominal pain, nausea, vomiting and diarrhea, and loss of appetite at a rural hospital. There were no other symptoms suggestive of malignancy. On examination, the patient was hemodynamically stable. Her abdomen was soft but distended. She had generalized abdominal tenderness, no peritonism, and an unremarkable per rectal examination. Her only medical history included morbid obesity and obstructive sleep apnea.
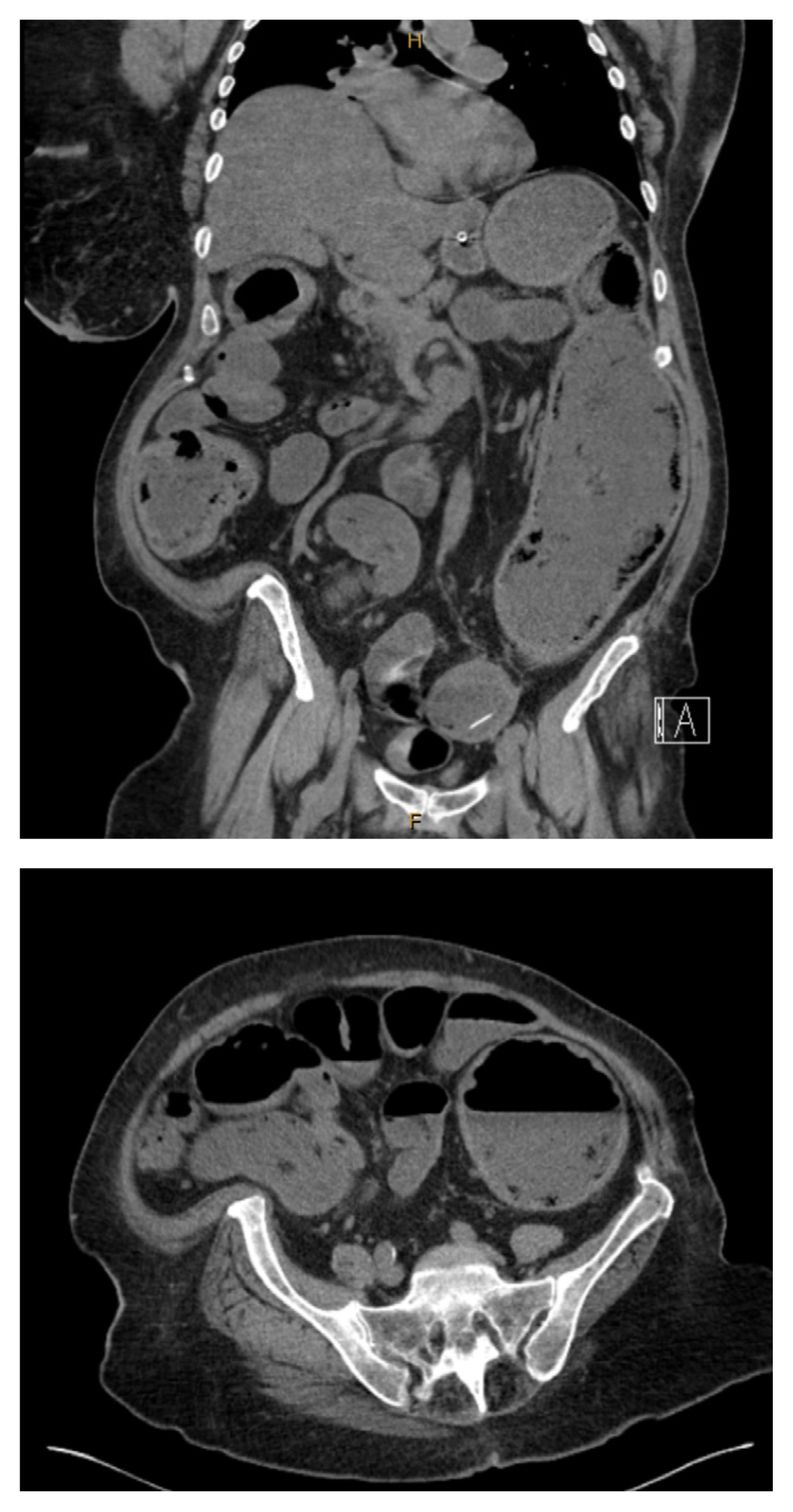
A nasogastric tube was placed, and a non-contrast computerized tomography (CT) scan (non-contrast due to a contrast shortage and Emergency Department protocol) was performed which demonstrated distension of the large bowel at the level of the rectosigmoid, with a region of aperture change at the level of the sigmoid (Figures 1A and 1B). Management continued for the suspected malignancy. The patient underwent a laparotomy and attempted Hartmann’s procedure the same day.
The index operation showed a large rectosigmoid mass tightly adherent to the terminal ileum, left ovary, and uterus. Large mesenteric nodes identified along the terminal ileum. An attempt was made to resect the adherent distal ileum from the mass however, this resulted in an enterotomy. In consultation with a specialist colorectal surgeon remotely via telephone, it was determined that the rectosigmoid tumor was unresectable, and that a diverting loop ileostomy should be formed. The firmly adherent distal ileum was resected and a functional side to side anastomosis was formed. A diverting loop ileostomy was formed with the proximal ileum. An appendicectomy was performed to decompress the large bowel which was followed by peritoneal lavage, and a mesenteric lymph node biopsy.
The patient’s postoperative course was complicated by the formation of an enterocutaneous fistula at the inferior edge of the laparotomy wound which had profuse small bowel output at Day 5. Associated with this were rising inflammatory markers (white cell count trending up to 19.8 with a C-reactive protein up to 192), tachycardia (heart rate between 110–120 bpm) and fevers up to 39.0°C, combined with contrast extravasation on repeat imaging (Figure 2). She was started on piperacillin/ tazobactam. A relook laparotomy was indicated, however, due to the high-risk surgical morbidity, the anesthetic team deemed a return to theater too high risk for a rural hospital and she was transferred to a metropolitan tertiary facility.
At Day 7, post index operation, the patient arrived at the tertiary center and underwent a relook laparotomy and Hartmann’s procedure. During this procedure, the findings were: (1) a significant volume of enteric contents in the abdominal cavity; (2) a leak due to failure of the small bowel anastomosis which was related to the loop ileostomy; (3) several interloop abscesses; and (4) a hard mass firmly adherent to the left side pelvic wall and retroperitoneum. On palpation of the proximal portion above the mass, there was suspicion of a foreign body potentially causing a focal perforation.
An attempt was made to perform medial to lateral mobilization, however it was difficult to mobilize the sigmoid mesentery off the retroperitoneum without potential left ureteric injury. The rectosigmoid mass was densely adherent, with no identifiable planes. The patient had intra-abdominal sepsis, and if this was a malignant process it would be another three to six months before safely being able to re-enter the abdominal cavity. A decision was made to perform a non-oncological colectomy without harvesting the inferior mesenteric artery lymph nodes. It was accepted that this would be a suboptimal oncological procedure, however, the etiology was more likely a non-malignant inflammatory process.
The mesentery was divided close to the bowel wall, the upper rectum was mobilized and divided just below the pelvic rim, stapled, and oversewn. A plastic bread clip (with an expiry date more than 20 years prior) was found within a pericolic abscess during the dissection (Figures 3A and 3B). The rectal stump was checked with flexible sigmoidoscopy to confirm there was no leak. The descending colon was decompressed and an end colostomy was formed. As for the anastomotic leak, it was taken down and resected, and an attempt was made to create an Abcarian (end-loop) ileostomy. However, due to the diameter of the small bowel, and the limited mobility as a result of adhesions, this was not possible. An end ileostomy, with the proximal end of the ileum, was formed.
This non-malignant obstruction was confirmed on histopathology (Figures 4, 5A and 5B). The ileostomy tissue was consistent with necrosis and acute serositis. The rectosigmoid tissue samples and associated lymph nodes showed multiple pericolic abscesses and perforation, with background changes of diverticulosis and diverticulitis. There was no granuloma formation, vasculitis, viral inclusions, parasites, dysplasia, or malignancy identified.
Outcome and Follow Up
The patient was initially managed in the intensive care unit where she required vasopressor support and was extubated on Day one post-operatively. The patient was transferred from the intensive care unit to the ward on Day three. She required a short period of parenteral nutrition due to ileus and her colostomy was complicated by mucocutaneous separation which was managed conservatively. All drains were removed on Day 12 postoperatively and she was transferred back to the regional center on Day 16. At the regional center, she developed high ileostomy output, which was treated with loperamide, Metamucil and dietary changes. She will be considered for reversal of end-colostomy, followed by a reversal of end-ileostomy at her next review at around six months from her last operative procedure.
Discussion
The causative natures behind LBOs can be divided into mechanical or functional, with mechanical largely being categorized into benign or malignant. Functional LBOs can be caused by systemic illnesses/disturbances such as sepsis, infective colitis, or medications such as narcotics [2]. The treatment of functional obstructions revolves around the management of the underlying systemic illness [2]. Mechanical obstructions can be caused by benign pathologies such as inflammatory bowel disease, diverticulitis, or volvulus [3]. A large proportion of patients that present with mechanical bowel obstructions, are due to malignancies which account for up to 50% of LBOs [4].
Malignant obstructions can be a surgical emergency if there is complete obstruction. These cases can require decompression of the bowel along with the removal of the obstruction if possible, or a surgical bypass if the tumor is unresectable [2].
A diagnosis of LBO can be made by a combination of clinical assessment and imaging. Imaging is commonly performed with either abdominal x-ray, CT, or contrast enema imaging. CT has been shown to be a highly effective imaging modality in the diagnosis of a LBO, with a sensitivity and specificity of 96% and 93%, respectively [3,5].
Management of malignant LBOs can be divided into nonsurgical and surgical. Nonsurgical measures focus on symptomatic relief such as gastric decompression with a nasogastric tube and antiemetics, with electrolyte optimization, and antibiotics if there is suspected perforation [1]. Surgical management can involve either stenting across the obstructing mass, or resection of the affected part of the bowel at times with a diverting stoma proximal to this obstruction [2].
This case highlights several interesting points relating to the presentation and surgical technique. Colorectal masses should be treated as a presumed cancer until proven otherwise. However, as indicated in this case, the importance of an in-depth assessment of the pre-operative imaging must be emphasized. The significance of preoperative workup in cases where there is minimal threat to perforation is high, and this should include contrast enhanced CT of both the bowel and chest, abdomen, and pelvis, to characterize the initial lesion and assess for metastases, and tests for tumor markers. In retrospect, the initial bread clip could be visualized on the non-contrast CT. If this had initially been noted, in combination with the pre-operative investigations, the operative course for this patient may have been significantly different. This brings into question, alternative surgical options, which may have entailed a diverting colostomy or double barrel ileostomy +/− cecostomy as a temporizing measure rather than the initial attempted oncological resection. Similarly, there is a question regarding the potential role of flexible sigmoidoscopy. Potentially performing a pre-operative flexible sigmoidoscopy may have allowed the surgical team to observe the inflammatory process, however there are limitations with this investigation and the potential inability to visualize the lumen would not have ruled out malignancy.
Conclusion
This case report of a LBO was initially thought to be attributed to a malignant obstructing, rectosigmoid tumor. This quickly changed from a case of a malignant obstruction, to one centered around a foreign body which had likely been present in the large bowel for a period of up to twenty years prior to the development of symptoms. To our knowledge, this is the only case report of an accidentally ingested foreign body causing delayed LBO.
Notes
Author Contributions
Conceptualization: MC, MJ and JK. Writing original draft: MC and MJ. Writing - review and editing: MC, MJ and JK.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding
There are no financial or non-financial interests that are direct or indirectly related to the work submitted for this publication.
Ethical Statement
This research did not involve any human or animal experiments. Patient consent obtained for publication.
Data Availability
All relevant data are included in this manuscript.