Introduction
Coagulation factor V (FV), also known as labile factor or proaccelerin, is a glycoprotein and is critical for both procoagulant and anticoagulant functions. FV acts as a cofactor of activated factor X in the prothrombinase complex, and accelerates the conversion of prothrombin to thrombin. Additionally, FV is associated with anticoagulation through the inactivation of factor VIII which is mediated by activated protein C [1,2]. FV is synthesized in the liver, and approximately 80% of the total content of FV is present in plasma, while the remaining 20% is retained in platelet alpha-granules [3,4].
FV deficiency is a coagulation disorder that can be congenital or acquired [5]. Acquired FV deficiency was first described in 1955. The overall incidence of FV deficiency is approximately 1 case per 1 million [2,3]. The incidence of acquired FV deficiency is estimated to be 0.09–0.29 per 1 million [6]. The acquired form generally results from the production of antibodies against FV following the administration of fresh frozen plasma (FFP) or antibiotics, autoimmune diseases, malignancies, or idiopathic formation of FV inhibitors [1,3]. Similar to congenital FV deficiency, prothrombin time (PT) and activated partial thromboplastin time (aPTT) are prolonged in acquired FV deficiency, where FV is at a low level. However, unlike the congenital form of the disorder, mixing tests for PT and aPTT are not corrected in the acquired FV form [1,2]. FV deficiency is divided into 3 grades according to the FV level: mild (FV level ≥ 10%), moderate (FV level < 10%), and severe (an undetectable FV level). Clinical symptoms often present as mucosal bleeding; but FV deficiency can cause bleeding anywhere in the body. In postoperative patients, the disorder may give rise to massive bleeding and result in mortality.
The treatment for the disorder involves FFP transfusion to ensure that the FV level is maintained at 20% or higher [1,2,4]. In addition, platelet transfusion may be another treatment option as FV is stored as a pre-active form in the alpha-granules of platelets [2,4]. In the case of the acquired form of the FV deficiency, immunosuppressive treatment is required to suppress antibody production against FV.
Herein, we report a case of acquired FV deficiency after administration of carbapenem antibiotics in an elderly patient.
Case Presentation
A 79-year-old male patient was admitted to the emergency room with abdominal pain. Abdominal computed tomography showed panperitonitis due to gastric ulcer perforation. The patient was taking an angiotensin II receptor antagonist (losartan) and a beta-adrenergic blocking agent (atenolol) for hypertension. In March 2021, he underwent an endoscopic gastroduodenoscopy for massive melena at another hospital and multiple active gastric ulcers were diagnosed. He was prescribed a 6-week course of proton pump inhibitor and his symptoms improved. No additional gastrointestinal bleeding was observed.
An emergency exploratory laparotomy was performed, and some soiling in the abdominal cavity was observed. As there was no clear perforation site, the surgery was completed after massive irrigation of the abdominal cavity and drain insertion. After the emergency operation, the patient was transferred to the intensive care unit. Hemodynamic instability developed, which required high dose of vasopressors. Combination therapy with piperacillin/tazobactam and teicoplanin was initiated for broad-spectrum antibiotic coverage for intra-abdominal infection. On the postoperative day (POD) 9 (POD #9), serum inflammatory markers were elevated, and the patient had a high fever. The piperacillin/tazobactam was subsequently changed to meropenem.
Blood coagulation tests were performed daily from admission. The first coagulation profile test was performed on the day before surgery, and all results were within the normal range. On POD #2, PT and aPTT were slightly prolonged to 17.6 seconds (ref.: 9.2–13.1 seconds) and 38.2 seconds (ref.: 26.8–40.6 seconds), respectively. From POD #3 to the POD #9 (the 1st day of administration of meropenem), PT showed a change from 13.9 to 15.3 seconds, while aPTT remained between 28.3 to 35.8 seconds. PT and aPTT remained slightly higher than normal levels but did not require coagulation factor supplementation or further examination. On the 4th day of meropenem administration (POD #13), PT and aPTT were prolonged to 33.0 seconds and 74.3 seconds, respectively. The prolongation of PT/aPTT was maintained even with vitamin K administration and FFP transfusion, and PT as well as aPTT were not corrected in the mixing test.
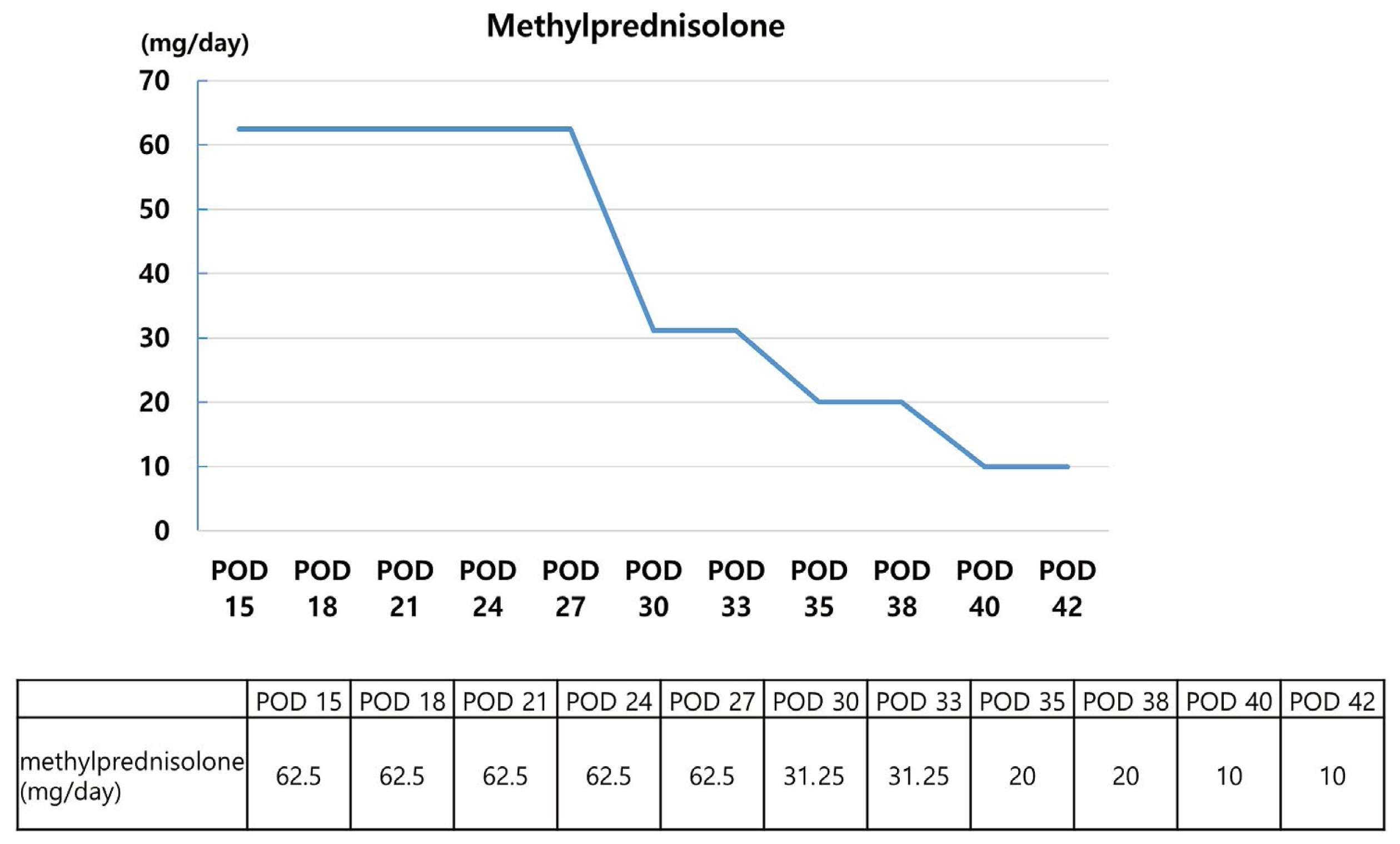
On POD #14, there was a hematological consultation on coagulopathy, and the factor assay including factor II, V, VIII, IX, and X was performed. The following day, acquired hemophilia due to FV deficiency was confirmed. Methylprednisolone administration (1 mg/kg/day) and FFP transfusion (6 cc/kg/day) were initiated immediately after the diagnosis. Meropenem, beta-lactam antibiotic, was considered as the responsible drug for FV deficiency. Meropenem was substituted with cefepime plus metronidazole to avoid beta-lactam antibiotics. A deep level of sedation (Richmond agitation sedation scale −4) was maintained to prevent blood pressure fluctuation because of the high risk of brain hemorrhage. Blood coagulation tests were performed daily, and FV assay was followed up once a week.
Even with high-dose intravenous steroid therapy, prolongation of PT/aPTT was not rapidly corrected. A hematologist was consulted and suggested that cyclophosphamide administration should be considered. However, the team decided to maintain the current treatment after a risk-benefit assessment of cyclophosphamide (including its immunosuppressive and delayed wound healing effect) was performed.
As the duration of mechanical ventilation therapy was prolonged, performing a tracheostomy could no longer be delayed. The surgery was carried out on POD #22 under general anesthesia. On this day, the patient’s PT and aPTT were 40.7 seconds and 109.8 seconds, respectively. Contrary to the blood coagulation test results, clinical symptoms were unclear. During the operation, there was no tendency to bleed and the estimated blood loss was < 5 cc. Subsequently, FFP transfusions were provided only when necessary. Methylprednisolone was reduced to 40 mg after administration at 1 mg/kg/day for 2 weeks, and the dose was subsequently reduced by half, every week. PT and aPTT began to show an improving trend from POD #25. Moreover, the measured value recovered and was in the normal value on POD #32 (PT: 13.5 seconds and aPTT: 27.0 seconds, respectively). FV levels observed at 1-week intervals were < 1% until the 3rd week, and reached 78.7% in the 4th week. Further, there were no abnormalities in other coagulation test results or clinical symptoms. The patient was therefore moved to a general ward on POD #38 and received additional treatment.
Discussion
This case presents the diagnosis and treatment process of a patient who developed a temporary deficiency of FV due to the production of antibodies against coagulation FV following administration of carbapenem antibiotics. The patient showed recovery of the FV titer and normalized coagulation test results after approximately 2 weeks of treatment.
In sepsis patients, abnormalities in blood coagulation tests are relatively common, therefore, conservative care is usually adopted. However, the team decided that consultant led treatment from experts was necessary, and conducted the related tests. Furthermore, the only drug that was added before the abnormalities occurred was meropenem, therefore, administration of this drug was halted early, and a change to the regimen, to other antibiotics, was made. Since variety of drugs have been reported to be responsible for FV deficiency, determining the cause and treatment can be difficult and time-consuming. However, in this case, meropenem was speculated to be the causative agent which enabled timely treatment.
The severity of bleeding due to FV deficiency is variable and the correlation between plasma FV levels and clinical manifestation is unclear [7]. Although the FV of this patient was < 1%, which corresponds to a relatively severe case, the patient experienced no events of bleeding and showed no tendency to bleed. Therefore, it was possible to avoid further complications by deciding not to administer a more potent immunosuppressive drug. Serious complications such as death from worsening sepsis may occur after the administration of immunosuppressives [8].
Acquired FV deficiency is a dangerous disorder that can lead to death due to massive bleeding. However, it is important to approach the treatment regimen knowing that good results can be obtained if administration of the causative drugs is halted, antibody production is inhibited, and coagulation factors are supplemented.