Surgical Techniques for Severe Liver Injury: A Comprehensive Review of Current Approaches and Advancements
Article information
Abstract
In abdominal trauma, the liver is the most injured organ and treatment is usually determined by hemodynamics. Severe liver injury with extensive parenchymal injury and uncontrollable bleeding may rapidly evolve into the lethal triad of death (acidosis, hypothermia, and coagulopathy), requiring damage control surgery (DCS). Damage control resuscitation for trauma treatment reduces the need for DCS by enabling rapid control of massive bleeding. Thus, definitive surgery can be completed in one operation. Despite the systematic application of damage control resuscitation, definitive surgery cannot be achieved in severe, and extensive liver injuries. Therefore, understanding, and acquiring damage control surgical techniques is necessary to achieve DCS for severe liver injury. The Western Trauma Association and the World Society of Emergency Surgery have proposed algorithms for the nonoperative and operative management of blunt hepatic trauma. The algorithms list several surgical skills, including electrocautery or argon beam, manual compression, perihepatic packing, the Pringle maneuver, liver suture, omental packing, selective hepatic artery ligation, balloon tamponade, hepatic vascular isolation, and the shunt operation. These techniques require a multidisciplinary approach and individual honing of skills by the surgeon. Trauma surgeons, even hepatobiliary surgeons, must practice damage control techniques in severe liver injury models (animals or cadavers).
Introduction
The most affected organ in abdominal trauma is the liver, and liver injury resulting from abdominal trauma presents a significant medical challenge. Severe liver injuries, characterized by extensive parenchymal damage and uncontrollable bleeding, can rapidly lead to the lethal triad of death—acidosis, hypothermia, and coagulopathy—necessitating immediate damage control surgery (DCS) [1]. The implementation of damage control resuscitation (DCR) in trauma treatment has revolutionized the approach to severe liver injury management by rapidly controlling massive bleeding and optimizing hemodynamic stability [2]. This has reduced the immediate need for DCS, allowing for definitive surgery during the initial operation, and improving patient outcomes [3–5].
Despite the benefits of DCR, achieving definitive surgery for severe and extensive liver injuries remains challenging due to the liver's complex anatomy and vital physiological functions. To address this, various surgical techniques have been proposed, including electrocautery or argon beam coagulation, manual compression, perihepatic packing, the Pringle maneuver, liver suture, omental packing, selective hepatic artery ligation, balloon tamponade, hepatic vascular isolation, and shunt operation [6–9]. The Western Trauma Association and the World Society of Emergency Surgery (WSES) have formulated algorithms for the operative management of blunt hepatic trauma, outlining these techniques to control bleeding and restore hemostasis [10,11].
To ensure the successful application of these techniques, a multidisciplinary approach and development of individual surgeon skills are crucial. Surgeons, including trauma and hepatobiliary specialists, benefit from practicing damage control techniques in severe liver injury models using animal models or cadavers to enhance proficiency and readiness. By critically reviewing the current state of surgical procedures and discussing emerging advancements, this review aims to contribute to the improvement of severe liver injury management, ultimately leading to better patient outcomes and reduced morbidity and mortality rates.
1. The lethal triad in severe liver injury
The lethal triad of death in severe liver injury is a life-threatening condition characterized by 3 critical factors: acidosis, hypothermia, and coagulopathy. Acidosis results from a decreased blood pH, impaired lactate clearance, and organ dysfunction. Hypothermia, a core body temperature below the normal range, often accompanies severe liver injuries, causing clotting abnormalities and exacerbating bleeding. Coagulopathy, the impaired function of blood to form clots, further contributes to uncontrolled bleeding and worsens the lethal triad itself in severe liver injury [12,13].
Addressing the lethal triad is vital for managing severe liver injuries effectively. Prompt interventions include optimizing fluid resuscitation and administering bicarbonate to correct acidosis. Active rewarming strategies such as warmed fluids and blankets help manage hypothermia. Coagulopathy requires the administration of blood products to restore clotting factors and control bleeding. Understanding and effectively managing the lethal triad can significantly improve patient outcomes in severe liver injury cases. Early recognition and targeted interventions are essential to mitigate its life-threatening consequences and reduce mortality rates [2].
2. Damage control surgery in liver injury
The choice to perform surgery on a patient with liver injury might be based on factors such as hemodynamic instability, a penetrating trauma with suspected secondary peritonitis, lack of response to resuscitation efforts, failed nonoperative management, or the presence of concurrent organ damage. Surgical treatment is usually required in high-grade liver injuries with major bleeding (WSES Grade III or IV; American Association for the Surgery of Trauma Grade IV and V) [6,11].
In these injuries, implementing DCR has transformed the management approach by rapidly controlling bleeding and stabilizing patients. DCR focuses on achieving hemostasis and restoring hemodynamic stability early on to prevent the lethal triad of death. The main components of DCR include permissive hypotension with restrictive fluid administration, early hemostatic resuscitation (transfusion of platelets: plasma: red blood cells in a high unit ratio (≥ 1:1:2) or reconstituted whole blood in a 1:1:1 unit ratio), correction of hypothermia, and acidosis, and rapid bleeding control by surgical and non-surgical techniques [1,14]. By promptly controlling bleeding, DCR reduces the need for immediate DCS, allowing for definitive surgery during the initial operation [5]. This approach streamlines treatment, potentially shortens hospital stays, and improves patient outcomes [2].
While DCR offers significant advantages, it has limitations. In cases of extensive liver injuries, the complexity of damage may surpass DCR's immediate potential, making definitive surgery unfeasible during the initial operation. Moreover, DCR may not address all underlying injuries, necessitating further interventions in the later stages of treatment. Additionally, severe liver injuries can involve damage to adjacent structures, requiring a multidisciplinary approach and careful surgical decision-making. By understanding these challenges and employing comprehensive damage control surgical techniques, trauma surgeons can optimize treatment strategies and improve patient outcomes.
3. Proposed algorithms for surgical management of liver injury
The Western Trauma Association and WSES algorithms offer valuable guidelines for managing hepatic trauma [10,11,15]. These guidelines emphasize the importance of assessing the patient's hemodynamic status and the severity of liver injury to guide treatment decisions. Both organizations share similarities in their guidelines in prioritizing nonoperative management with or without angioembolization for stable patients with low-grade injuries, focusing on close monitoring and imaging. For hemodynamically unstable patients or those with high-grade liver injuries, both organization’s algorithms also highlight the importance of DCR techniques in achieving hemostasis and avoiding the lethal triad of death, emphasizing perihepatic packing in severe liver injuries. The 2 algorithms generally offer a similar overview of the various surgical procedures.
As both organization’s algorithms underscore the significance of DCR in facilitating definitive surgery, the principal contrast arises from their focus on specific techniques. The WSES algorithm accentuates the utilization of combined endovascular techniques and damage control laparotomy for uncontrolled major bleeding. Furthermore, the WSES algorithm advocates for the consideration of laparoscopic interventions to reduce invasiveness in surgical procedures, especially in the presence of suspected intra-abdominal injuries in minor liver trauma or the context of delayed complications.
Choosing the most appropriate of the 2 algorithms may depend on factors such as available resources, a surgeon’s expertise, and patient-specific characteristics. Integrating the strengths of both algorithms and adapting the management approach to individual cases can lead to improved outcomes in hepatic trauma management.
4. Damage control surgical techniques for severe liver injury
Severe liver injuries resulting from abdominal trauma pose significant challenges to trauma surgeons due to extensive parenchymal damage and uncontrolled bleeding. In such critical scenarios, immediate intervention is essential to stabilize the patient and prevent further deterioration. Damage control surgical techniques are vital in achieving hemostasis and controlling bleeding, and enabling subsequent definitive surgery.
4.1. Incision
For the initial incision, consider a midline laparotomy [7], which offers limited exposure to the posterior and lateral liver areas. Depending on the specific anatomical region and the extent of liver injury, additional incisions may become necessary. To enhance access to posterolateral liver injuries, a right subcostal incision to branch off from the initial laparotomy may be required [8]. Additionally, in cases where access to the atriocaval shunt is necessary, a median sternotomy may be warranted [16]. This approach provides excellent exposure to the posterior and lateral aspects of the liver. However, when definite packing is essential for controlling liver bleeding, this supplementary incision may compromise the tamponade effect of the packing. In situations where severe liver injuries necessitate damage control packing, early recognition is crucial, and it's advisable to preserve the abdominal wall and ligaments to facilitate more effective packing.
4.2. Electrocautery or argon beam coagulation
Electrocautery and argon beam coagulation are frequently employed to achieve hemostasis by cauterizing bleeding vessels and tissues. These techniques are instrumental in managing superficial liver injuries and controlling minor bleeding [10]. Roughly 80–85% of patients undergoing surgery can effectively address their liver injury using straightforward surgical methods like applying local hemostatic agents, electrocoagulation, superficial sutures, or drainage [8]. The remaining 15–20% of cases necessitate more intricate surgical approaches.
4.3. Manual compression and perihepatic packing
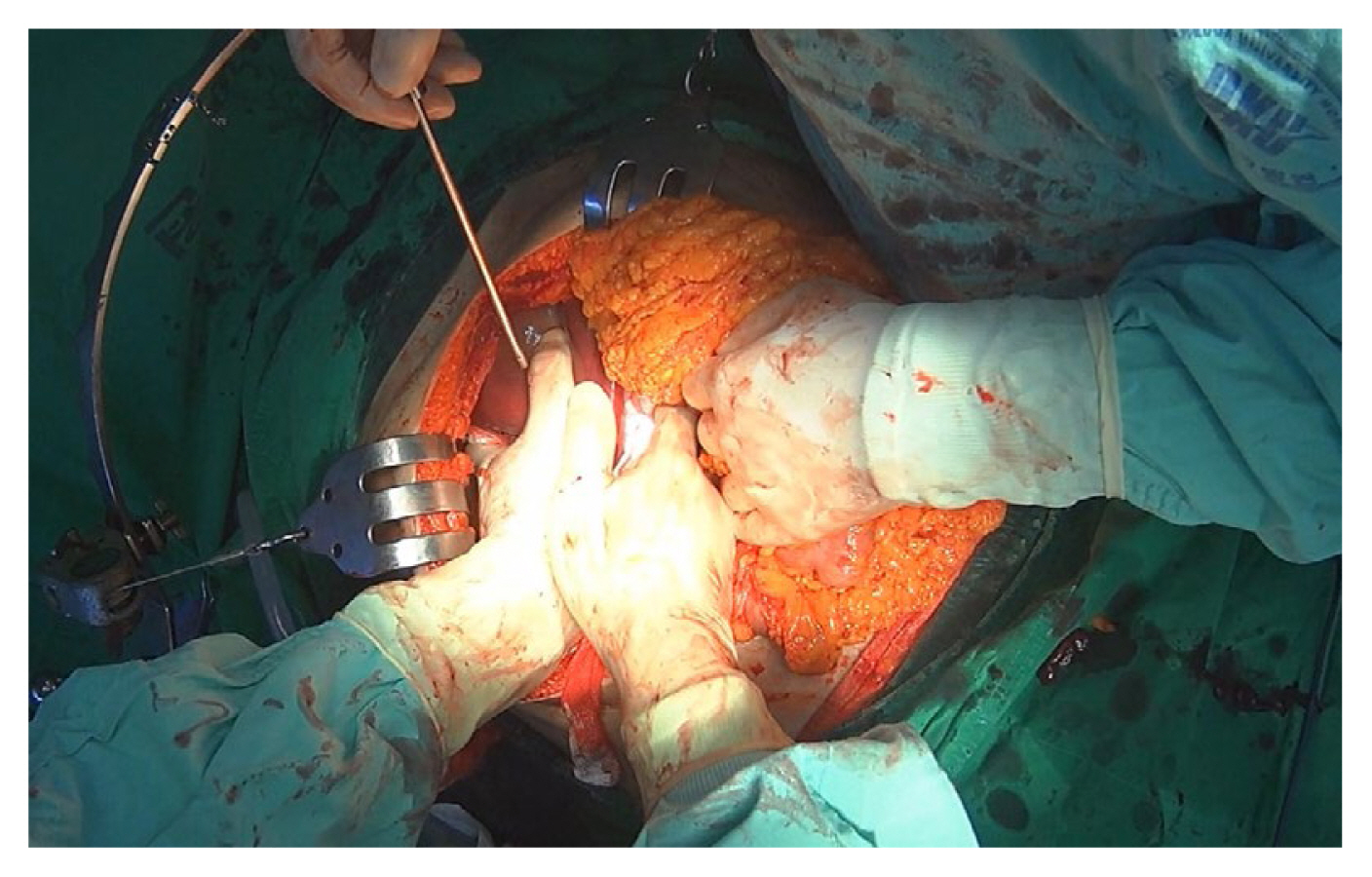
Manual compression involves the direct application of pressure on bleeding sites to control hemorrhage temporarily (Figure 1). Bimanual compression is applied by concomitantly compressing the right and left sides of the injury site [6,7]. Periodic assessment is exclusively focused on hemostasis confirmation. Upon successful hemostasis, transitioning to packing is advocated. In cases of ineffectiveness, consideration may be given to performing the Pringle maneuver. It is imperative to abstain from application of direct pressure to the injured site. Subsequent damage to the parenchyma has the potential to induce disruption and exacerbate bleeding.
Perihepatic packing entails the use of sterile gauze to pack the liver, achieving hemostasis and controlling bleeding in cases of severe injury and uncontrolled hemorrhage [8,17]. Laparotomy packs are strategically positioned superior and inferior to the liver's bleeding site, creating a “hepatic sandwich” (Figure 2). The packing material is applied in layers, ensuring that direct pressure is not exerted on the injury site. After at least 10 minutes, the packing's hemostatic effect should be assessed. If there is no ongoing bleeding and the patient remains hemodynamically stable, the pack should be carefully removed. In cases where bleeding persists after pack removal, implementing the Pringle maneuver may be considered. In complex scenarios involving disseminated intravascular coagulation, uncontrolled bleeding, expanding subcapsular hematomas, retrohepatic inferior vena cava (IVC) injury, and similar circumstances, the intervention should be concluded with definitive packing. In perihepatic packing, discrete packs are positioned in the posterior paracaval, lateral, anterior, and posteroinferior compartments [17]. The formation of well-structured packing configurations is pivotal in achieving an effective tamponade. To optimize exposure, liver mobilization is facilitated by releasing the falciform and coronary ligaments [7]. Conversely, the preservation of hepatic ligament integrity amplifies the tamponade's efficacy. As such, it is advised to refrain from undertaking mobilization routinely.
A retrohepatic hematoma that remains contained and stable should be managed conservatively without surgical intervention. In cases of hematoma expansion or leakage, employing controlled packing alone is the preferred operative approach. A hazardous maneuver involves liver retraction for enhanced visualization, especially in patients with hepaticocaval junction injuries [18]. The placement of pads on the liver's superior surface is discouraged. Consequently, subhepatic pads applied with upward compression approximate the liver fracture, achieving compression of the right hepatic lobe against the diaphragm. In retrohepatic injuries, the liver should be compressed posteriorly against the IVC and hepatic veins. No packing materials should be positioned behind the liver. If this approach proves effective, exploration is unnecessary. It's imperative to exercise caution, as overly tight packing may lead to IVC occlusion and may compromise venous return, potentially resulting in hypotension. Notably, it's crucial to recognize that packing is ineffective in controlling major arterial bleeding. Therefore, postoperative hepatic artery angiography should be performed (Figure 3) [8]. Several studies have reported varying rates of post-DCS angiography to manage arterial bleeding (52–62%), with some studies showing a lower mortality (12% vs. 36%) in Grade IV–V hepatic injury patients who undergo angioembolization compared with patients who did not undergo [19–22]. Two main indications for postoperative angioembolization in high-grade liver injuries are:

Hepatic artery angiography after damage control surgery with perihepatic packing (laparotomy pads, white arrow) shows contrast leakage (black arrow) from the right hepatic artery.
(1) after primary operative hemostatic control in stable patients with computed tomography (CT)-scan-confirmed bleeding; and
(2) as an adjunctive hemostatic control for uncontrolled arterial bleeding despite emergency laparotomy [11].
The decision to remove packing should be contingent upon the correction of hypothermia, acidosis, and coagulopathy, typically within a timeframe of 36 to 72 hours [23]. Prolonged utilization of packing materials elevates the susceptibility to intraabdominal sepsis, with packs left in place for over 3 days exhibiting an 83% incidence of perihepatic sepsis [24]. Premature pack removal carries the potential for an increased risk of rebleeding or the need for re-packing. Nicol et al [25] documented a higher re-bleeding rate linked to the early removal of packs at 24 hours. Interestingly, whether packing was maintained for 2 or 3 days, there were no observable disparities in complication rates. Caruso et al [23] advocated for the removal of packs within a 36- to 72-hour timeframe, as early removal (within 36 hours) was associated with a heightened likelihood of requiring re-packing due to recurrent bleeding.
Diverse strategies have been outlined to mitigate the risk of rebleeding during pack removal. One approach involves moistening the gauze with saline before removal. Another technique entails the placement of a nonadherent plastic drape directly on the hepatic surface, followed by the positioning of laparotomy pads on the top [26,27]. Additionally, an absorbable mesh may be applied over the hepatic surface before packing. Notably, this mesh is permanently retained when the packs are removed [8].
4.4. Pringle maneuver
The Pringle maneuver temporarily occludes the hepatic inflow by clamping the hepatoduodenal ligament with a vascular clamp and Rummel tourniquet [28]. This technique reduces blood flow to the liver providing improved visibility for surgeons, and facilitates bleeding control during liver injury repair. Under normothermic conditions, the accepted duration for safe cross-clamping has traditionally been limited to 15–20 consecutive minutes [29]. However, this technique raises significant concerns regarding ischemia and reperfusion injury. To date, the definitive maximum safe occlusion time remains undetermined. Furthermore, the debate persists regarding the superiority of continuous versus intermittent Pringle maneuver. In the context of hepatectomy for liver tumors, intermittent Pringle maneuver during liver resection is generally considered safe for a period of up to 120 minutes [30]. Nevertheless, the applicability of intermittent Pringle maneuver in trauma cases remains a subject of inquiry, as it may lead to increased bleeding during clamp removal or may prolong the overall operation time. Notably, one study reported the routine use of the continuous Pringle maneuver for 30–60 minutes without encountering adverse outcomes [31]. Another study supported the continuous application of the Pringle maneuver for up to 75 minutes without associated morbidity [32]. Consequently, in trauma cases, it may be advisable to limit the continuous Pringle maneuver to a duration not exceeding 60 minutes.
It is well established that in cases of uncontrolled bleeding, despite the implementation of the Pringle maneuver, the possibility of IVC or hepatic vein injury should be considered. Nevertheless, it is crucial to acknowledge the potential oversight of hepatic artery anomalies. One frequently encountered anomaly is the emergence of the right hepatic artery as the primary branch of the superior mesenteric artery, and an accessory left hepatic artery may have its origin in the left gastric artery, providing perfusion to the left lateral segment [33,34]. Failure to detect these anomalies during the Pringle maneuver may result in the persistence of bleeding.
4.5. Liver suturing
Suturing is employed to repair liver lacerations and achieve hemostasis. Various suture techniques such as simple continuous sutures or figure-of-eight sutures are utilized based on the extent and location of the injury [6,8,33]. Superficial defects may be effectively managed through the application of simple interrupted sutures. In more profound lacerations, the utility of figure-of-eight or mattress sutures is observed. To achieve a tension-free closure in deeper tissue layers, the use of 0–0 to 2–0 chromic sutures is advocated. The utilization of blunt-tipped needles is endorsed for their capacity to mitigate parenchymal trauma and facilitate ease of manipulation. It is advisable to introduce the needle into the liver parenchyma at a perpendicular 90-degree angle to reduce the risk of parenchymal disruption during knot tying.
Implementation of the pledgeted sutures, complemented by polytetrafluoroethylene pledgets, in conjunction with the horizontal mattress suture method, utilizing absorbable stitching is shown in Figures 4A and 4B. This procedural preference not only safeguards against inadvertent liver capsule damage but also ensures proficient compression and precise tissue approximation [35]. Particularly for relatively extensive lacerations, it is possible to perform horizontal mattress sutures on uninterrupted pledget sheets without cutting (Figure 4C). This technique can facilitate efficient compression across extensive damage, leading to the successful achievement of hemostasis.
(A) Deep liver laceration in the right subhepatic aspect (after cholecystectomy). (B) Horizontal mattress sutures on interrupted pledgets. (C) Horizontal mattress sutures on uninterrupted pledget sheets.
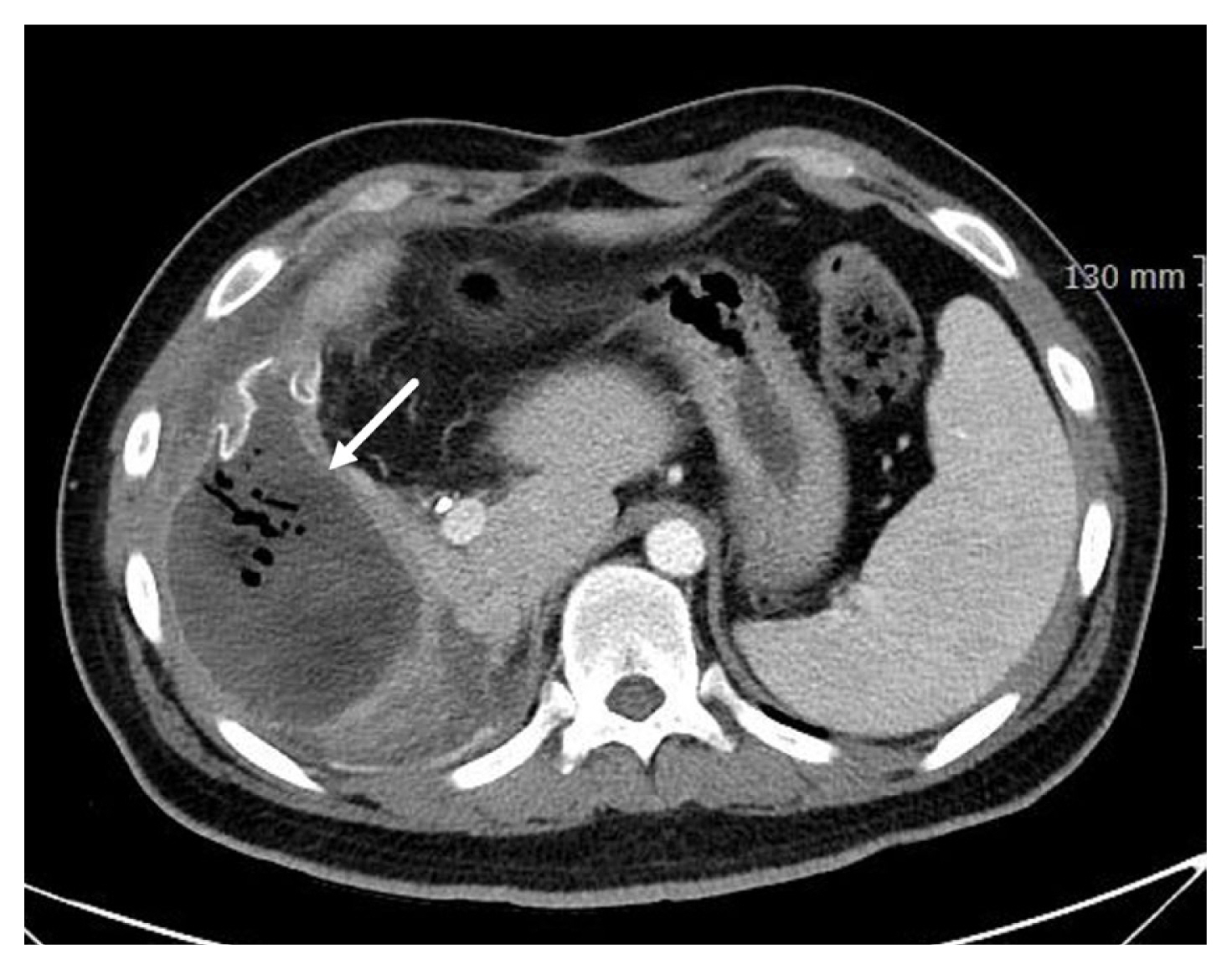
Liver suturing is accompanied by inherent risks, such as intrahepatic abscesses, hematoma formation, or hemobilia, as consequences of deep suture placement [6,8]. The diagnostic approach typically involves the use of a CT scan, and effective management can often be achieved through percutaneous drainage or angiographic embolization (Figure 5). Notably, the incidence of abscess formation is frequently associated with the utilization of pledgeted sutures [36]. Severe liver injuries can lead to a contaminated environment due to bile leakage at the laceration site. In cases of infection, this area can transform into a challenging-to-treat refractory abscess, especially when pledgets and foreign bodies are present. Considering hepatorrhaphy with absorbable polyglycolic acid pledgets is a viable approach for managing severe liver injuries [37]. Nevertheless, it is important to note that the use of polyglycolic acid pledgets in liver surgery remains controversial. The imposition of overly tight sutures in liver surgery poses a notable risk of hepatic necrosis [6,8]. The liver's tendency to swell following surgery accentuates this concern. As a precautionary measure, it is advised that sutures are loose rather than having tight tension.
4.6. Omental packing
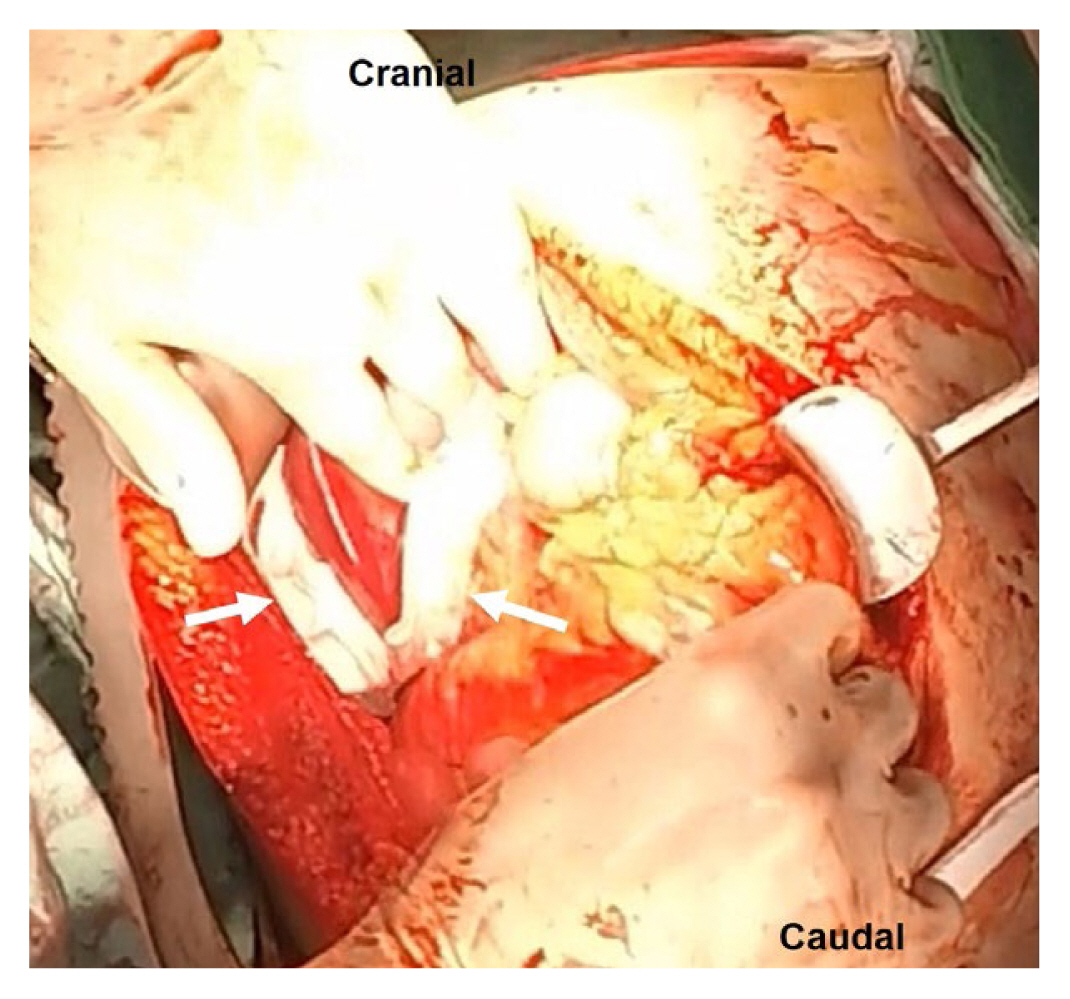
The greater omentum, with its rich blood supply, serves as an effective packing material to cover and compress liver injuries, aiding in hemostasis and promoting tissue healing [6–8,27,38,39]. The omentum not only serves as a hemostatic agent to control oozing but also provides a rich source of macrophages which can reduce the risk of subsequent sepsis [40]. Omental packing is employed to address more profound and broader hepatic lacerations or hepatotomy sites (Figure 6). Following achieving hemostasis through direct ligation or repair, omental packing is utilized primarily to manage minor venous bleeding. Mobilization of the omentum is performed while preserving the integrity of the right gastroepiploic vascular pedicle. The pedicle flap is introduced into the disrupted area. Omental packing can be combined with other techniques, particularly in cases of extensive liver injury. The omental pack is covered with a nonadherent plastic drape, and perihepatic packs are positioned above it to facilitate compression [8,41]. The tamponade effect achieved with omental packing may offer advantages over most direct hemostatic methods.
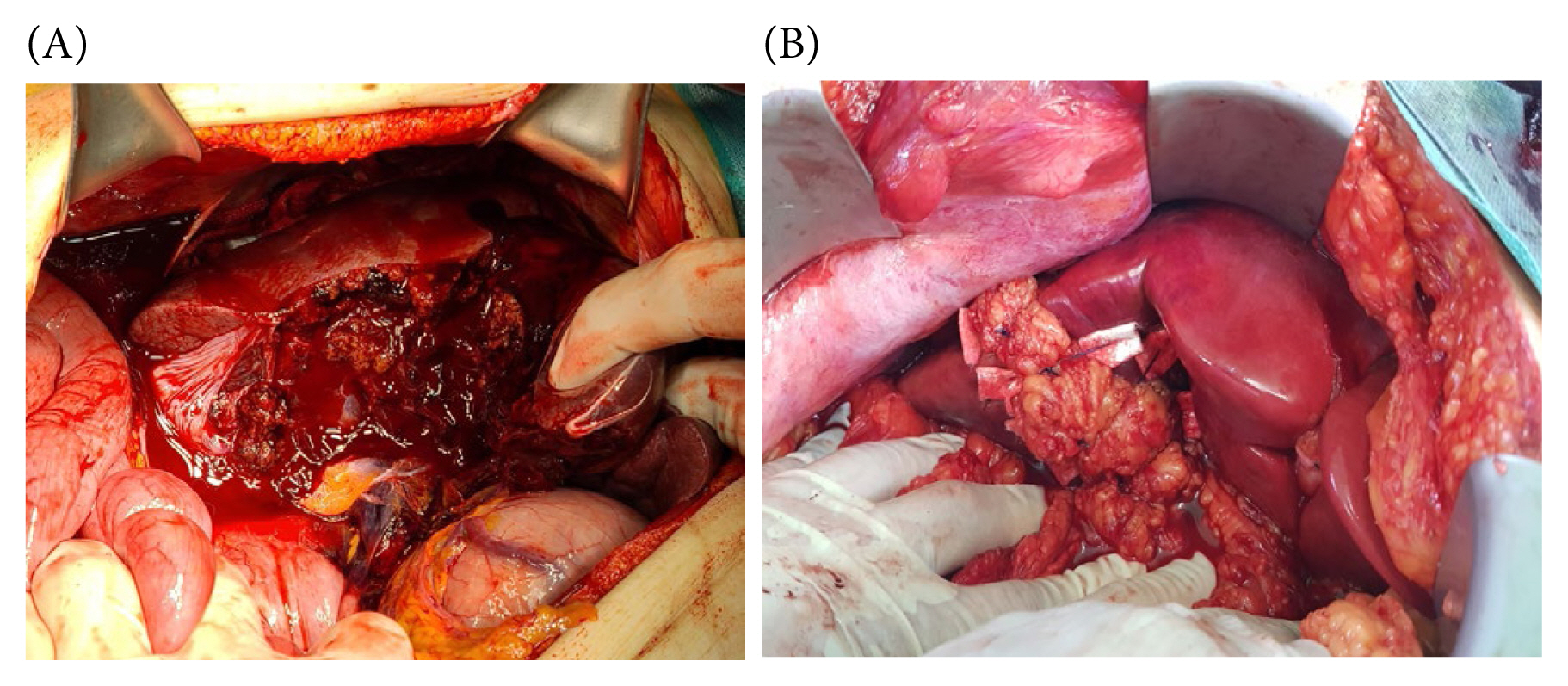
(A) Extensive hepatic laceration involving a right liver. (B) Omental packing in a split liver injury.
Omental packing may present certain drawbacks, notably the potential for hepatic necrosis or abscess formation (Figure 7) [42,43]. This risk can be attributed to the excessive tightness of sutures securing the omentum to the liver, leading to omental strangulation and subsequent partial hepatic necrosis.
4.7. Hepatotomy (tractotomy) with selective vascular ligation
Hepatotomy with selective ligation plays a pivotal role in the management of bleeding arising from deep lacerations [6,7]. Hepatotomy procedures can be executed along the laceration path using diverse techniques such as a linear stapler, manual finger fracture methods, or an electrothermal bipolar vessel sealing system commonly known as the LigaSure device [8]. More recently, the adoption of stapling devices has been recommended. After parenchymal fracture, interventions involving direct repair are carried out., such as suture ligation, or the application of clips to visible ducts and vessels. Occasionally, the argon beam coagulator, hemostatic agents, or gauze are employed to control persistent hemorrhagic oozing [27]. Hepatotomy involving the finger fracture technique is continued until bleeding vessels are identified and successfully managed [44]. However, hepatotomy procedures may inadvertently transect the healthy parenchyma, resulting in a notable reduction in parenchymal volume and triggered additional bleeding, particularly in cases of coagulopathy. This sequence of events can potentially initiate a vicious cycle of uncontrollable bleeding.
4.8. Balloon tamponade
An alternative to tractotomy is the use of a tamponade with a balloon catheter. A suitable choice for this purpose includes a Sengstaken-Blakemore tube (primarily designed for esophageal varices), a large Foley catheter, or a custom-made balloon fashioned from a Penrose drain or a surgical glove [8,27,45]. Many surgical experts recommend employing a Penrose drain placed over a red Robinson catheter and secured at both ends [46]. The “balloon” over the Robinson catheter is inserted into the tract and inflated with saline. It is crucial to ensure that approximately 2–3 cm of the catheter protrudes from both ends of the tract. In deep and large-diameter penetrating injuries, a plastic bowel bag can be introduced through the tract to manage the situation, and several laparotomy pads are then placed into the bag, creating intrahepatic tamponade [47]. After hemorrhage control has been achieved, perihepatic packing is carried out, and the clamped catheter is exteriorized through the lateral abdominal wall. Postoperative angiography is advisable, and during angiography, deflation may be necessary. The balloon catheter is typically removed within 24 to 48 hours [6]. However, if active bleeding persists, repositioning of the balloon catheter or resorting to a hepatotomy with selective vascular ligation may be deemed necessary [48].
4.9. Shunting maneuvers
Shunt operations involve creating vascular conduits to reroute blood flow and reduce blood loss in complex vascular injuries or when primary repair is not feasible. In retrohepatic IVC injuries, juxtahepatic venous trauma, or severe uncontrolled liver injury, temporary hemorrhage control can be attained through total hepatic vascular isolation or the use of an atriocaval shunt [6,7,27]. These approaches are considered bridging therapies. Nevertheless, it is advisable to prioritize the employment of an atriocaval shunt, which offers a 10% to 30% survival rate [6,16,29,49], over total hepatic vascular isolation which is associated with less favorable survival outcomes [50,51].
Total hepatic vascular isolation encompasses the clamping of both suprahepatic and infrahepatic portions of the IVC and the porta hepatis (Figure 8) [6,52]. As this abrupt interruption of venous return can potentially induce cardiac arrest, it is imperative to prioritize supraceliac aortic clamping or the application of a Zone 1 resuscitative endovascular balloon occlusion of the aorta (REBOA). Supraceliac aortic clamping involves employing a DeBakey aortic clamp to occlude the aorta above the celiac axis [8]. Due to the presence of hematoma and active bleeding, clamping the IVC is a highly challenging endeavor, and carrys a substantial risk of exacerbating the existing injury. Another viable option for addressing retrohepatic injuries is the implementation of a venovenous bypass, necessitating cannulation of the femoral and axillary veins [29,53].
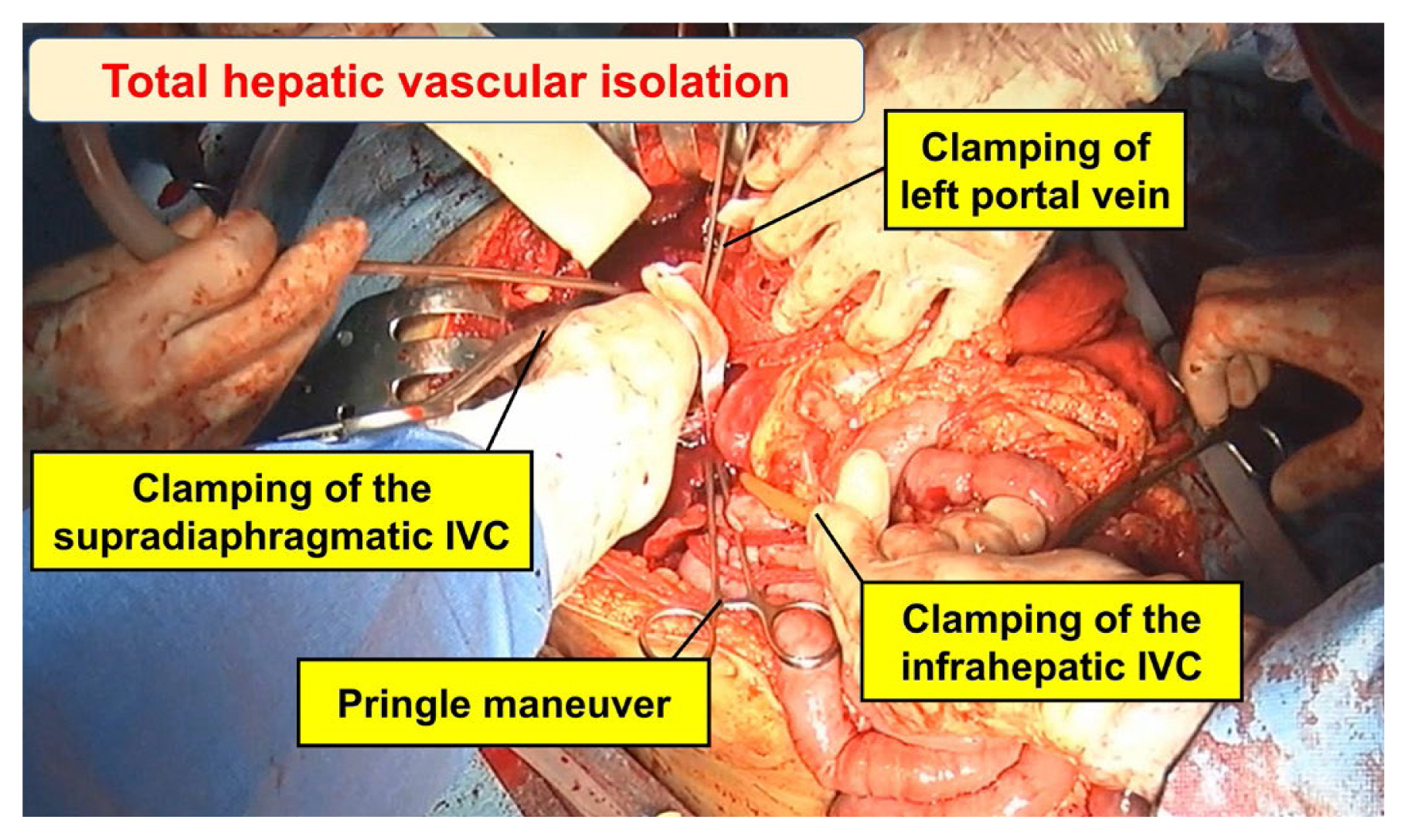
Total hepatic vascular isolation in an extensive left hepatic laceration with retrohepatic IVC injury. Clamping of the supradiaphragmatic IVC injury was performed via a transdiaphragmatic incision.
IVC = inferior vena cava.
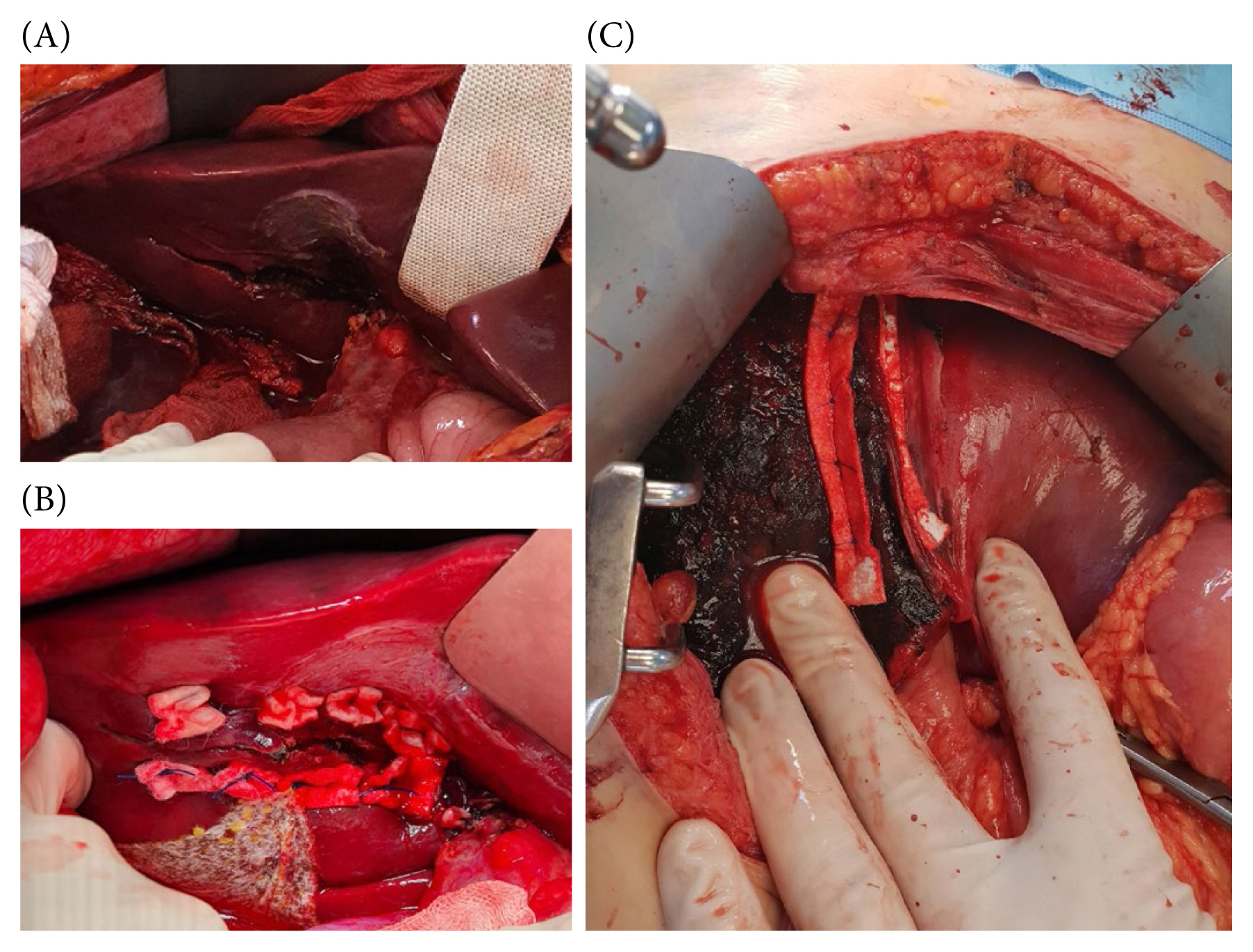
The primary objective of the atriocaval shunt is to divert all venous blood flow away from the infrahepatic IVC, channeling it through the shunt, and into the right atrium (Figure 9) [6,52,54]. This procedure necessitates prompt execution with all requisite equipment readily available and requires a median sternotomy. Typically, a 34 to 36 French chest tube or an 8 French endotracheal tube serves as the shunt conduit. Additional perforations are made in the tube at the level of the right atrium, ensuring an essential complete bypass. Once this definitive bypass is established, the shunt creates a well exposed surgical field for repairing the injured IVC through direct suturing. It is critical for perihepatic packing to serve as a tamponade for the shunt procedure to be effective. It is worth noting that many surgeons have moved away from employing the atriocaval shunt due to the associated technical complexities and suboptimal outcomes. The outcome is significantly influenced by the expertise of the surgical team and the timing of the shunt placement [16]. It is imperative to consider this approach at an early stage, particularly before the development of substantial coagulopathy and severe hypothermia in patients with moribund status.
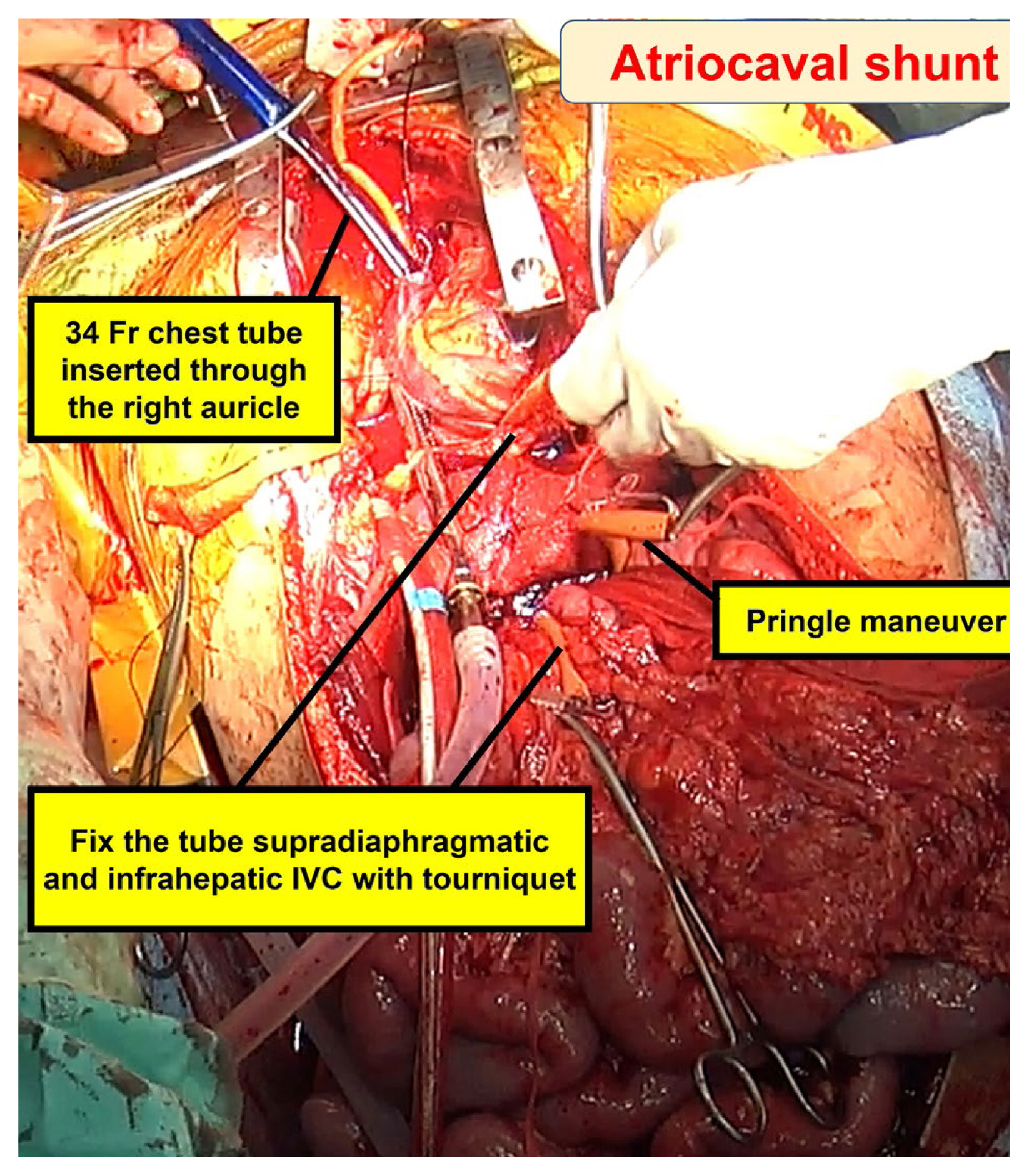
Atriocaval shunt was performed by exposing the heart through a median sternotomy.
IVC = inferior vena cava.
Additional shunting techniques have emerged as alternatives. The retroperitoneal cavoatrial shunt involves the insertion of a tube into the right atrium through a venotomy in the infrarenal IVC, which is in contrast to the atriocaval shunt [55]. Choi et al [56] reported a case in which a retrohepatic IVC injury was successfully treated by employing atriocaval shunt placement through a transdiaphragmatic incision without sternotomy or thoracotomy. On the other hand, the retrohepatic vena cava balloon shunt entails the introduction of a dedicated balloon catheter into the retrohepatic IVC through the saphenofemoral junction [57,58]. It is noteworthy that these techniques remain primarily documented through rare case reports.
4.10. Endovascular alternatives
As presented in the WSES algorithm, endovascular techniques for retrohepatic bleeding have been proposed as alternative strategies [11]. In managing hemodynamically compromised patients, the REBOA can be employed as an interim measure, bridging more decisive procedures to achieve hemorrhage control [59]. Applying the resuscitative endovascular balloon occlusion of the vena cava (REBOVC) for retrohepatic IVC injury management in a swine model resulted in a significant decrease in both blood loss and the time to fatality [60]. The placement of 1 REBOA and 2 REBOVCs is performed under fluoroscopic guidance to access the thoracic aorta, suprahepatic, and infrahepatic IVC [61]. Furthermore, a combination involving one REBOA and one REBOVC positioned within the thoracic aorta and retrohepatic IVC has been explored [62]. This specific arrangement employing REBOVCs enhances intraoperative visualization and the potential for subsequent repair. It is noteworthy that these approaches are still in the experimental phase. A case in which 2 preoperative REBOVCs were utilized for an infrarenal IVC injury, followed by an emergency laparotomy is shown in Figure 10.
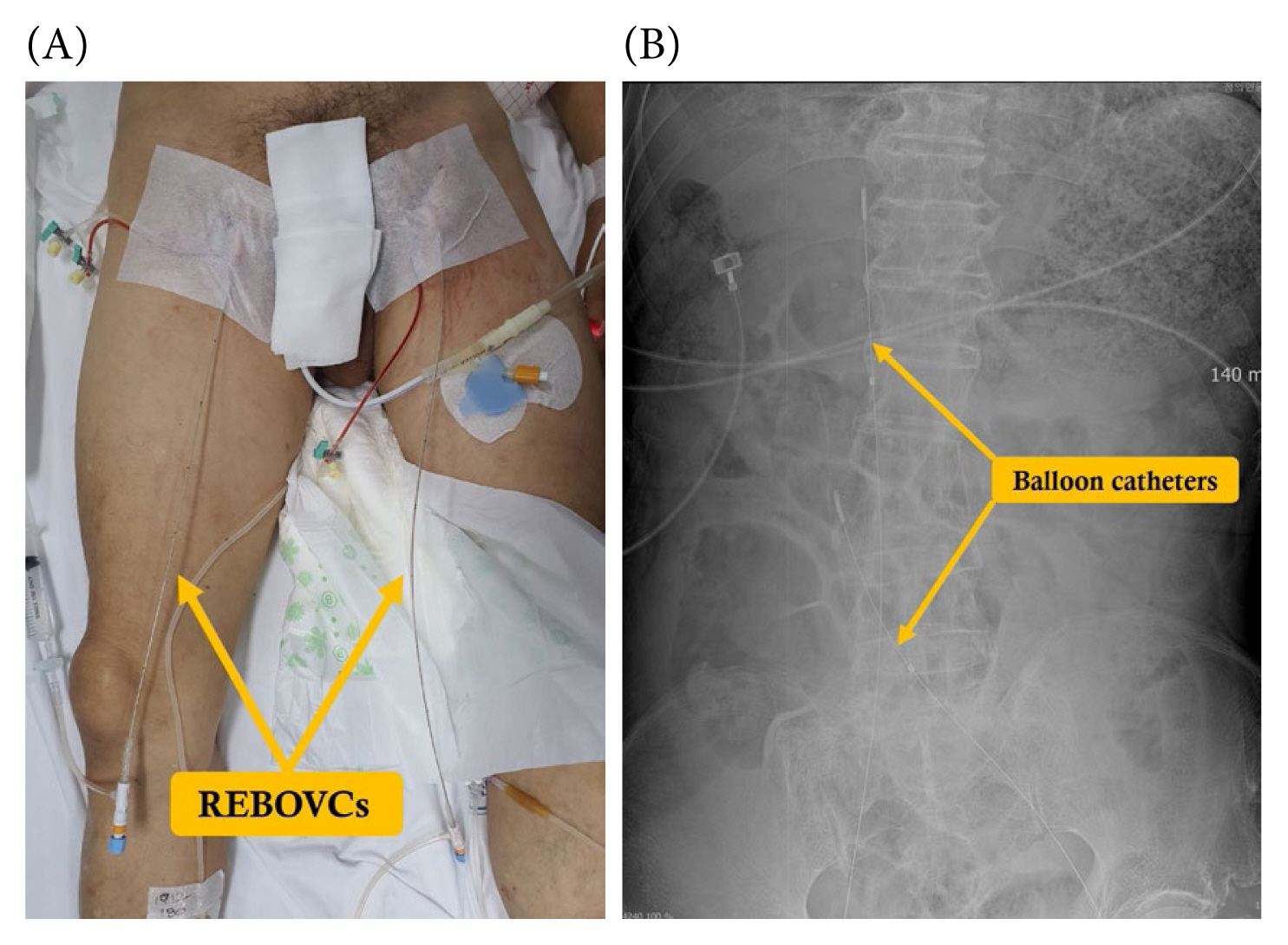
(A) Performance of the preoperative REBOVCs through bilateral common femoral veins. (B) The abdominal X-ray image demonstrates 2 REBOVCs, highlighting the positioning of balloon catheters in both the suprarenal vena cava and above the confluence of the common iliac veins, intended to manage infrarenal vena cava injury.
REBOVC = resuscitative endovascular balloon occlusion of the vena cava.
4.11. Hepatic resection
In unstable patients and during DCS, it is advisable to avoid anatomic hepatic resection whenever possible. Nonetheless, in cases where anatomic resection is warranted, the nonanatomic approach is considered the safer and more practical option [6,11]. It is important to note that if substantial liver laceration due to the injury is already present, further resection and debridement may be required to address retrohepatic bleeding. The safety of both anatomic and nonanatomic resections when conducted by experienced surgeons have been reported [10,63,64]. Polanco et al [64] shared their insights based on patients who underwent hepatic resection during their initial operation, with a reported morbidity rate of 30% and a mortality rate of 17.8%.
4.12. Hepatic transplantation
For cases involving liver avulsion or total crush injury, where complete hepatic resection is deemed necessary, the option of hepatic transplantation has been documented with favorable outcomes. Through a rigorous review of 28 relevant articles, Plackett et al [65] conducted an extensive analysis of 31 liver transplant patients. The results of their study demonstrated an overall survival rate of 61.3%, concurring with an equivalent graft survival rate at the 1-year time point. A retrospective investigation based on data from the European Liver Transplant Registry underscores the significance of an ISS score below 33 when selecting recipients to ensure that futile procedures are avoided [11,66]. Jeon et al [67] published the first report in Korean, detailing a successful hepatic transplantation on Hospital Day 28. The case involved a 65-year-old male with a Grade IV liver injury who was initially managed nonoperatively with angioembolization but subsequently progressed to hepatic failure.
These damage-control surgical techniques offer valuable options for trauma surgeons to address severe liver injuries promptly and effectively. The systematic application and adaptability of these techniques to the patient's specific condition play a crucial role in achieving damage control during the initial surgery. However, it is essential to consider the individual patient's clinical status and the extent of the liver injury to determine the most appropriate combination of techniques to optimize patient outcomes.
5. Multidisciplinary approach and skill development
In managing severe liver injuries, a multidisciplinary collaboration among various medical specialties is essential for optimizing patient care [22]. Trauma surgeons, hepatobiliary specialists, anesthesiologists, interventional radiologists, and critical care experts must collaborate to provide comprehensive and timely treatment. This collaborative approach ensures that all aspects of patient care, from initial assessment to postoperative management, are optimized for the best possible outcomes. Interventional radiologists play a crucial role in performing procedures like transcatheter arterial embolization to control bleeding, complementing the efforts of surgeons, and enhancing treatment effectiveness [10,11].
To achieve proficiency in managing severe liver injuries, trauma or hepatobiliary surgeons require continuous skill development and specialized training. Participating in courses, workshops, and simulation-based training helps refine techniques and decision-making abilities. Simulation tools, including high-fidelity simulators and virtual reality platforms, provide valuable opportunities for surgeons to practice various scenarios and gain confidence in managing critical liver injuries. Practicing damage control surgical techniques in severe liver injury models, such as animal models or cadavers, can significantly contribute to skill development. These models offer realistic simulations, enabling surgeons to improve their approach, practice specific maneuvers, and enhance decision-making skills in a controlled environment. Mentorship and proctorship programs provide valuable guidance, allowing young surgeons to learn from experts.
Conclusion
Damage control surgical techniques play a crucial role in the management of severe liver injuries, where rapid control of bleeding, and stabilization of patients are paramount. The systematic application of DCR has revolutionized trauma treatment, allowing for timely management of massive bleeding and reducing the need for immediate DCS. Multidisciplinary collaboration remains essential in providing comprehensive patient care, with close cooperation between trauma surgeons, hepatobiliary specialists, interventional radiologists, and critical care experts.
The implications of advancements in surgical techniques and ongoing research extends beyond the field of hepatic trauma. By refining skill development, through training and utilizing animal models and cadavers, surgeons can optimize their expertise in managing complex liver injuries. Ultimately, this multidisciplinary approach and dedication to skill improvement can significantly improve patient outcomes, elevating the standard of care, and transforming the management of severe liver injuries.
Notes
Conflicts of Interest
The author has no conflicts of interest to disclose.
Funding
None
Data Availability
All relevant data are included in this manuscript.