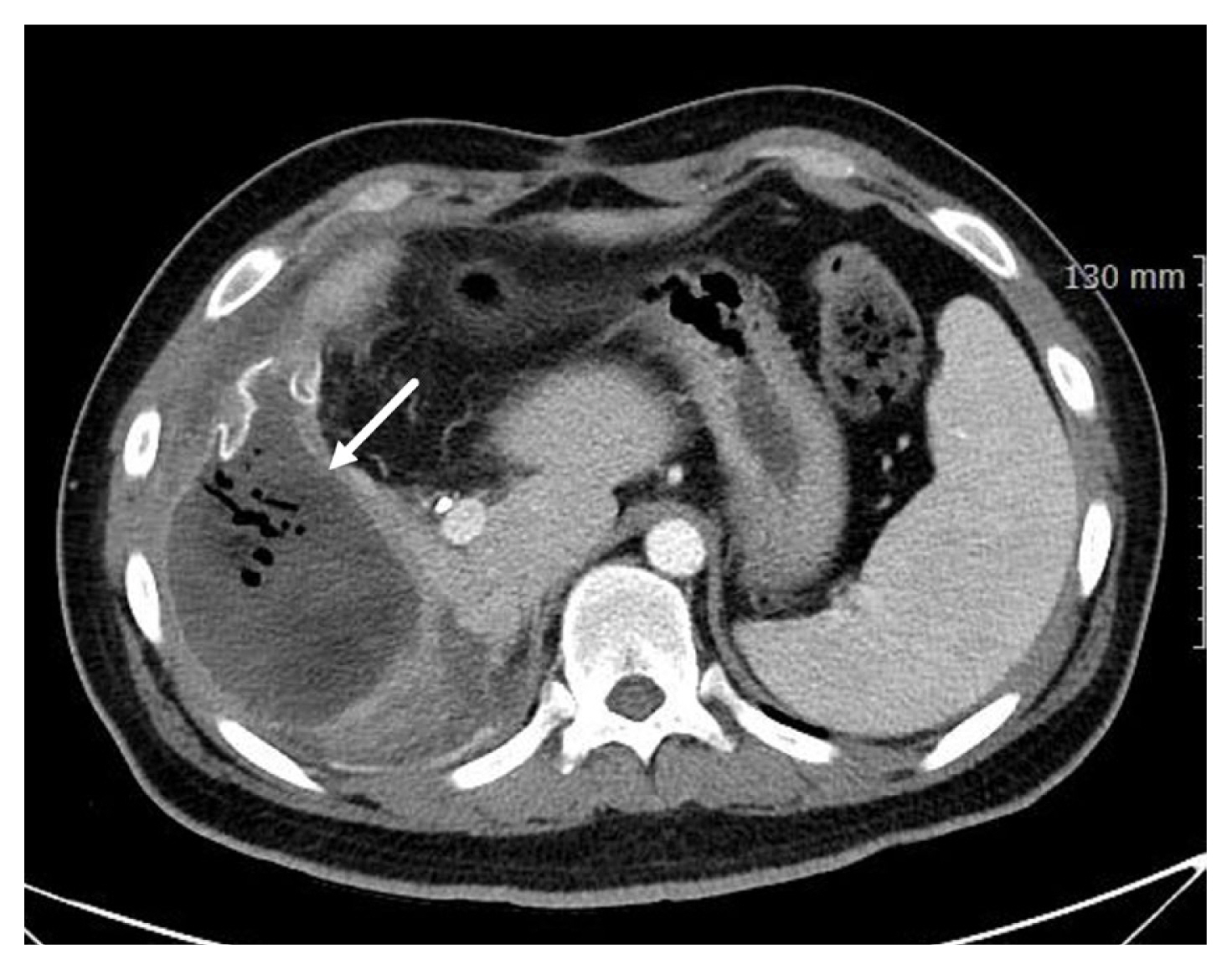
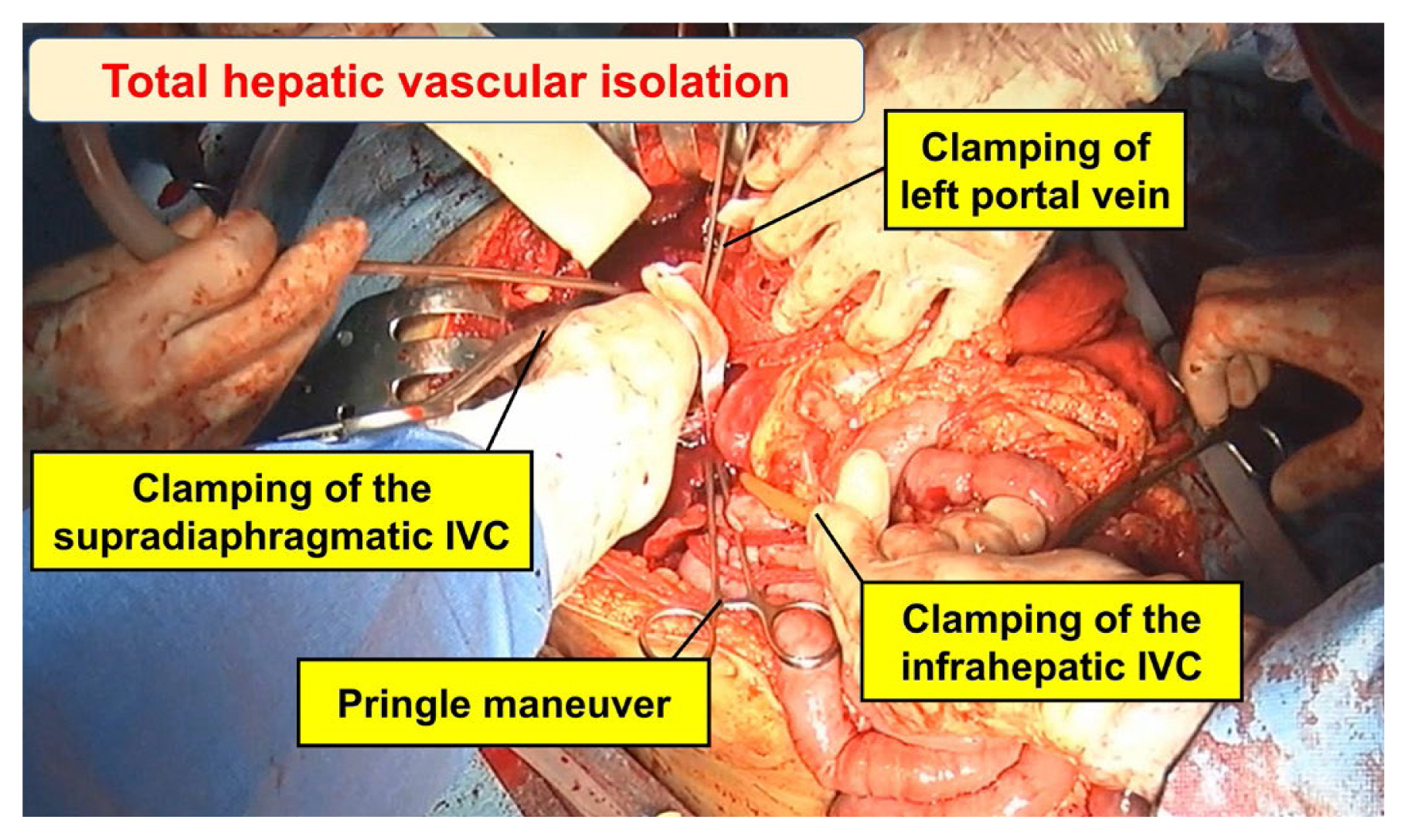
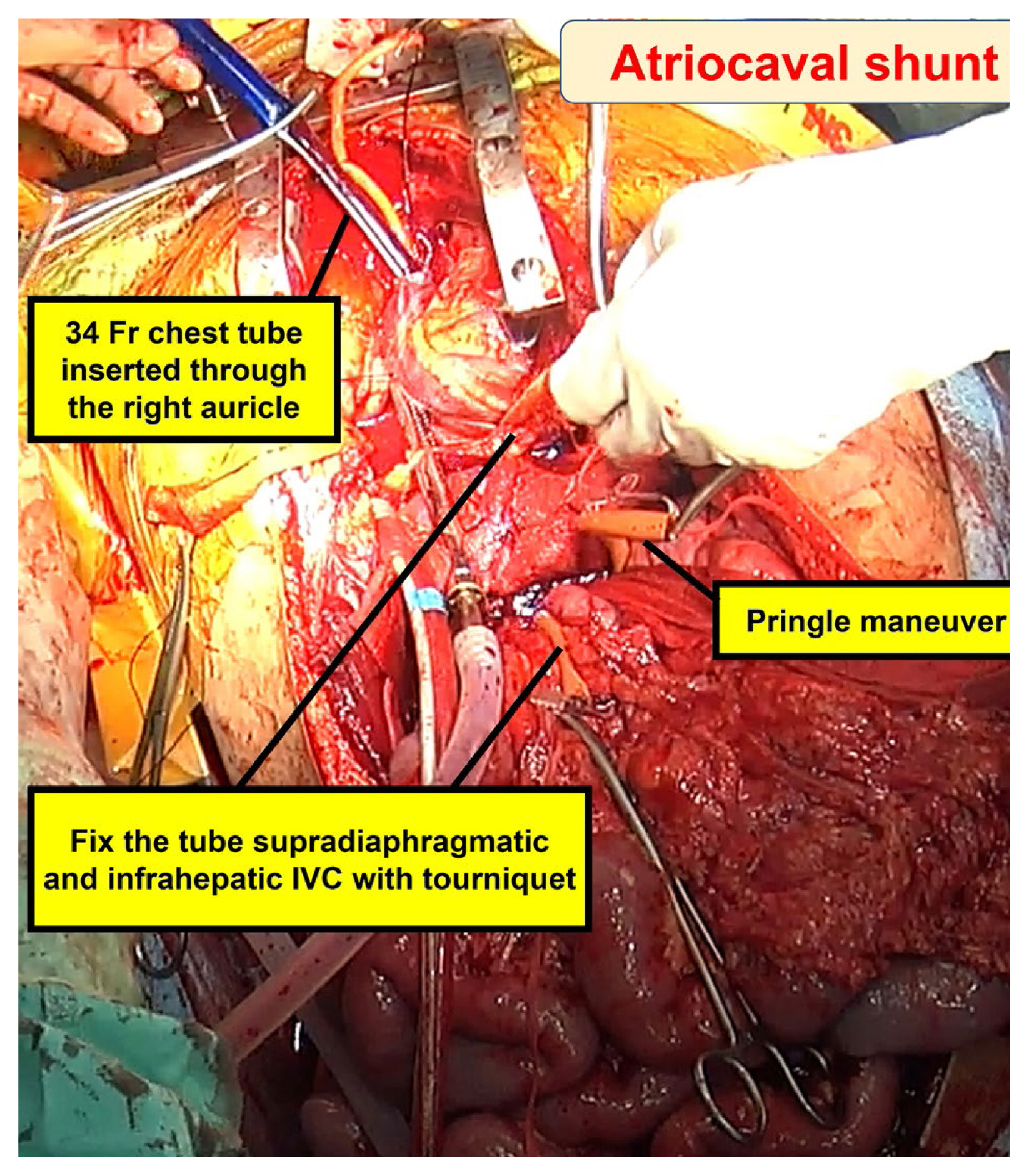
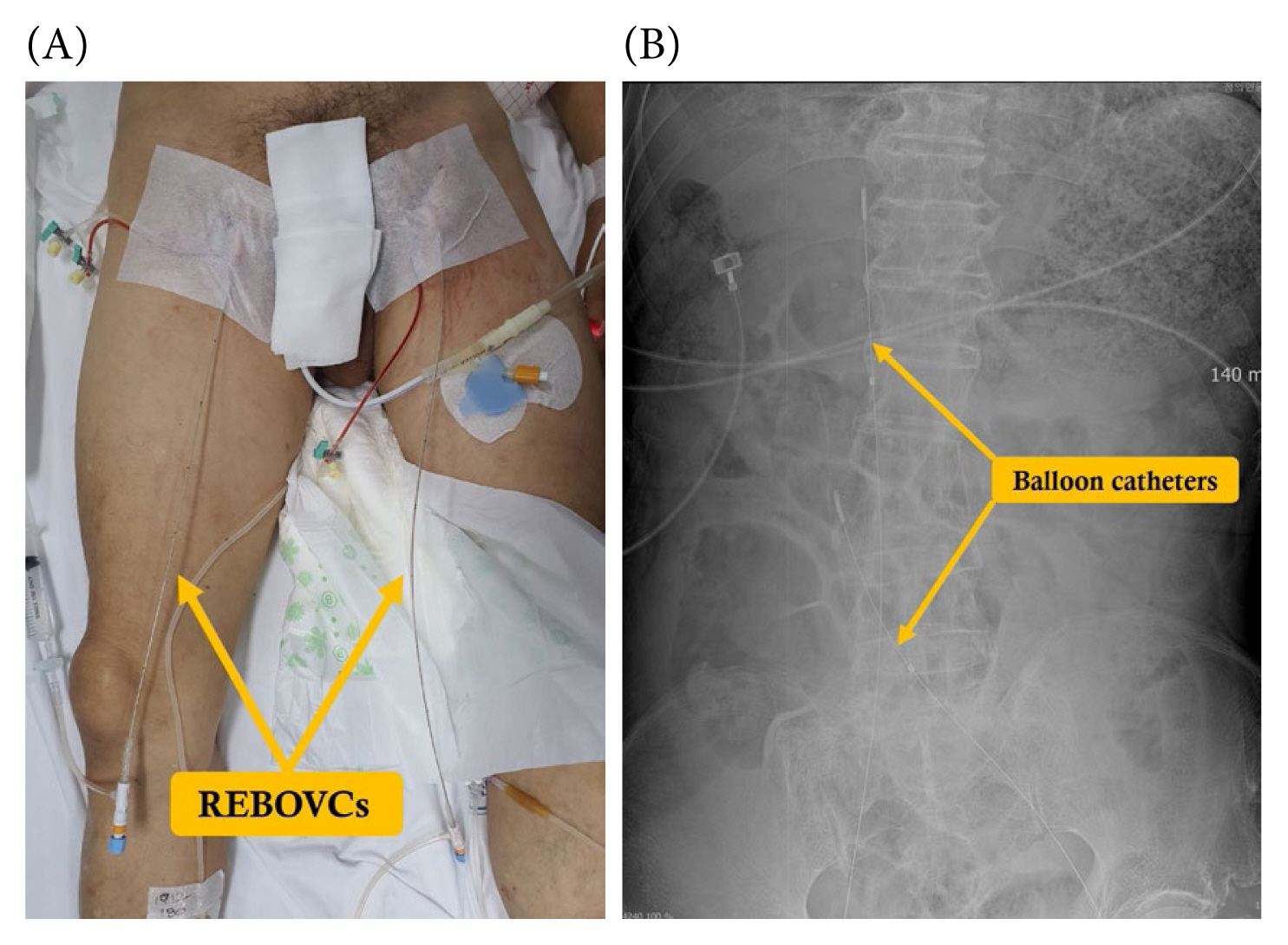
3. Jansen JO, Thomas R, Loudon MA, Brooks A. Damage control resuscitation for patients with major trauma. BMJ 2009;338:b1778.


4. Duchesne JC, Kimonis K, Marr AB, Rennie KV, Wahl G, Wells JE, et al. Damage control resuscitation in combination with damage control laparotomy: a survival advantage. J Trauma 2010;69(1):46–52.


6. Bruns B, Kozar R. Liver and Biliary Tract. Feliciano DV, Mattox KL, Moore EE. editors. Trauma. 9th ed. New York (NY): McGraw-Hill Medical; 2021.
7. Parikh MS, Brundage SI, Pachter HL. Liver Injury. Asensio JA, Trunkey DD. editors. Current therapy of trauma and surgical critical care. 2nd ed. Philadelphia (PA): Elsevier; 2016.

8. Inaba K, Warriner ZD, Vogt K. Liver and biliary tract injuries. Demetriades D, Inaba K, Velmahos G. editors. Atlas of surgical techniques in trauma. 2nd ed. Cambridge (UK): Cambridge; 2020.

9. Azoulay D, Salloum C, Lim C. Surgery of the inferior vena cava. Cham (Switzerland): Springer; 2017.
10. Kozar RA, Feliciano DV, Moore EE, Moore FA, Cocanour CS, West MA, et al. Western Trauma Association/critical decisions in trauma: operative management of adult blunt hepatic trauma. J Trauma 2011;71(1):1–5.


11. Coccolini F, Coimbra R, Ordonez C, Kluger Y, Vega F, Moore EE, et al. Liver trauma: WSES 2020 guidelines. World J Emerg Surg 2020;15(1):24.


12. Cohen MJ, Christie SA. Coagulopathy of trauma. Crit Care Clin 2017;33(1):101–18.


14. Camazine MN, Hemmila MR, Leonard JC, Jacobs RA, Horst JA, Kozar RA, et al. Massive transfusion policies at trauma centers participating in the American College of Surgeons Trauma Quality Improvement Program. J Trauma Acute Care Surg 2015;78(6 Suppl 1):S48–53.


15. Kozar RA, Moore FA, Moore EE, West M, Cocanour CS, Davis J, et al. Western Trauma Association critical decisions in trauma: nonoperative management of adult blunt hepatic trauma. J Trauma 2009;67(6):1144–9.


17. Baldoni F, Di Saverio S, Antonacci N, Coniglio C, Giugni A, Montanari N, et al. Refinement in the technique of perihepatic packing: a safe and effective surgical hemostasis and multidisciplinary approach can improve the outcome in severe liver trauma. Am J Surg 2011;201(1):e5–14.


18. Letoublon C, Amariutei A, Taton N, Lacaze L, Abba J, Risse O, et al. Management of blunt hepatic trauma. J Visc Surg 2016;153(4 Suppl):33–43.


20. Misselbeck TS, Teicher EJ, Cipolle MD, Pasquale MD, Shah KT, Dangleben DA, et al. Hepatic angioembolization in trauma patients: indications and complications. J Trauma 2009;67(4):769–73.


21. Johnson JW, Gracias VH, Gupta R, Guillamondegui O, Reilly PM, Shapiro MB, et al. Hepatic angiography in patients undergoing damage control laparotomy. J Trauma 2002;52(6):1102–6.


23. Caruso DM, Battistella FD, Owings JT, Lee SL, Samaco RC. Perihepatic packing of major liver injuries: complications and mortality. Arch Surg 1999;134(9):958–63.


26. Feliciano DV, Mattox KL, Burch JM, Bitondo CG, Jordan GL Jr. Packing for control of hepatic hemorrhage. J Trauma 1986;26(8):738–43.


27. Feliciano DV. Liver Packing. Ivatury RR. editors. Operative techniques for severe liver injury. Cham (Switzerland): Springer; 2015.

28. Feliciano DV, Pachter HL. Hepatic trauma revisited. Curr Probl Surg 1989;26(7):453–524.


29. Pachter HL. Prometheus bound: evolution in the management of hepatic trauma--from myth to reality. J Trauma Acute Care Surg 2012;72(2):321–9.

30. Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg 1999;134(5):533–9.


33. Lucas CE, Ledgerwood AM. Treatment of Liver Injuries: An Overview. Ivatury RR. editors. Operative techniques for severe liver injury. Cham (Switzerland): Springer; 2015.

34. Dudeja V, Ferrantella A, Fong Y. The Liver. Townsend CM, Beauchamp RD, Evers BM, Mattox KL. editors. Sabiston: textbook of surgery: the biological basis of modern surgical practice. 21st ed. Philadelphia (PA): Elsevier; 2022: p.1433.
35. Mulholland TL, See WA. Pledgeted sutures for parenchymal compression facilitate partial nephrectomy. Br J Urol 1998;81(4):630–3.


37. Nakamura T, Shimizu Y, Watanabe S, Hitomi S, Kitano M, Tamada J, et al. New bioabsorbable pledgets and non-woven fabrics made from polyglycolide (PGA) for pulmonary surgery: clinical experience. Thorac Cardiovasc Surg 1990;38(2):81–5.


38. Stone HH, Lamb JM. Use of pedicled omentum as an autogenous pack for control of hemorrhage in major injuries of the liver. Surg Gynecol Obstet 1975;141(1):92–4.


39. Fabian TC, Stone HH. Arrest of severe liver hemorrhage by an omental pack. South Med J 1980;73(11):1487–90.


40. Bluett MK, Woltering E, Adkins RB. Management of penetrating hepatic injury. A review of 102 consecutive patients. Am Surg 1984;50(3):132–42.

41. McHenry CR, Fedele GM, Malangoni MA. A refinement in the technique of perihepatic packing. Am J Surg 1994;168(3):280–2.


42. Sefczek RJ, Lupetin AR, Beckman I, Dash N. CT appearance of omental packs. Radiology 1985;156(2):472.


43. Vas W, Bilotta W, Sundaram M, Wolverson M, Markivee C. Computed tomography of omental liver packs. J Comput Assist Tomogr 1988;12(4):592–4.


45. Ozdogan M, Ozdogan H. Balloon tamponade with Sengstaken-Blakemore tube for penetrating liver injury: case report. J Trauma 2006;60(5):1122–3.


46. Poggetti RS, Moore EE, Moore FA, Mitchell MB, Read RA. Balloon tamponade for bilobar transfixing hepatic gunshot wounds. J Trauma 1992;33(5):694–7.


47. Ong AW, Kelly R, Jeremitsky E, Cortes V, McAuley CE, Rodriguez A. Liver packing: a variation of an old technique. J Trauma 2007;63(6):1405–6.


48. Demetriades D. Balloon tamponade for bleeding control in penetrating liver injuries. J Trauma 1998;44(3):538–9.


49. Richardson JD. Changes in the management of injuries to the liver and spleen. J Am Coll Surg 2005;200(5):648–69.


50. Carrillo EH, Spain DA, Miller FB, Richardson JD. Intrahepatic vascular clamping in complex hepatic vein injuries. J Trauma 1997;43(1):131–3.


51. Nicoluzzi JE, Von Bahten LC, Laux G. Hepatic vascular isolation in treatment of a complex hepatic vein injury. J Trauma 2007;63(3):684–6.


52. Trunkey DD, Pharaon KS. Juxtahepatic venous injuries: emergency measures, definitive control, and atriocaval shunts. Ivatury RR. editors. Operative techniques for severe liver injury. Cham (Switzerland): Springer; 2015.

53. Biffl WL, Moore EE, Franciose RJ. Venovenous bypass and hepatic vascular isolation as adjuncts in the repair of destructive wounds to the retrohepatic inferior vena cava. J Trauma 1998;45(2):400–3.


54. Lam L, Tadlock MD, Demetriades D. Inferior Vena Cava. Demetriades D, Inaba K, Velmahos G. editors. Atlas of surgical techniques in trauma. 2nd ed. Cambridge (UK): Cambridge; 2020.

55. Lucas CE. The Evolution of Liver Injury Diagnosis and Treatment in the Past 50 Years. Panam J Trauma Crit Care Emerg Surg 2014;3(3):124–31.

57. Pilcher DB, Harman PK, Moore EE Jr. Retrohepatic vena cava balloon shunt introduced via the sapheno-femoral junction. J Trauma 1977;17(11):837–41.


58. McAnena OJ, Moore EE, Moore FA. Insertion of a retrohepatic vena cava balloon shunt through the saphenofemoral junction. Am J Surg 1989;158(5):463–6.


59. Beyer CA, Johnson MA, Galante JM, DuBose JJ. Zones matter: Hemodynamic effects of zone 1 vs zone 3 resuscitative endovascular balloon occlusion of the aorta placement in trauma patients. Injury 2019;50(4):855–8.


60. Reynolds CL, Celio AC, Bridges LC, Mosquera C, O’Connell B, Bard MR, et al. REBOA for the IVC? Resuscitative balloon occlusion of the inferior vena cava (REBOVC) to abate massive hemorrhage in retrohepatic vena cava injuries. J Trauma Acute Care Surg 2017;83(6):1041–6.


61. Rezende-Neto JB, Al-Kefeiri G, Strickland M, Prabhudesai V, Rizoli SB, Rotstein O. Three sequential balloon catheters for vascular exclusion of the liver and aortic control (one REBOA and two REBOVCs): a hemorrhage control strategy in suprahepatic vena cava injuries.

62. Ordoñez CA, Herrera-Escobar JP, Parra MW, Rodriguez-Ossa PA, Puyana JC, Brenner M. A severe traumatic juxtahepatic blunt venous injury. J Trauma Acute Care Surg 2016;80(4):674–6.


63. Peitzman AB, Marsh JW. Advanced operative techniques in the management of complex liver injury. J Trauma Acute Care Surg 2012;73(3):765–70.


64. Polanco P, Leon S, Pineda J, Puyana JC, Ochoa JB, Alarcon L, et al. Hepatic resection in the management of complex injury to the liver. J Trauma 2008;65(6):1264–9. discussion 1269–70.


65. Plackett TP, Barmparas G, Inaba K, Demetriades D. Transplantation for severe hepatic trauma. J Trauma 2011;71(6):1880–4.


66. Krawczyk M, Grąt M, Adam R, Polak WG, Klempnauer J, Pinna A, et al. Liver transplantation for hepatic trauma: a study from the European liver transplant registry. Transplantation 2016;100(11):2372–81.

67. Jeon S, Yu B, Lee GJ, Lee MA, Park Y, Cho J, et al. Liver transplant after severe liver trauma: the first report in a Korean adult. Exp Clin Transplant 2023;21(7):619–22.